p75NTR: an enhancer of fenretinide toxicity in neuroblastoma
- PMID: 23314735
- PMCID: PMC3581726
- DOI: 10.1007/s00280-013-2071-7
p75NTR: an enhancer of fenretinide toxicity in neuroblastoma
Abstract
Objective: Neuroblastoma is a common, frequently fatal, neural crest tumor of childhood. Chemotherapy-resistant neuroblastoma cells typically have Schwann cell-like ("S-type") morphology and express the p75 neurotrophin receptor (p75NTR). p75NTR has been previously shown to modulate the redox state of neural crest tumor cells. We, therefore, hypothesized that p75NTR expression level would influence the effects of the redox-active chemotherapeutic drug fenretinide on neuroblastoma cells.
Methods: Transfection and lentiviral transduction were used to manipulate p75NTR expression in these cell lines. Sensitivity to fenretinide was determined by concentration- and time-cell survival studies. Apoptosis incidence was determined by morphological assessment and examination of cleavage of poly-ADP ribose polymerase and caspase-3. Generation and subcellular localization of reactive oxygen species were quantified using species- and site-specific stains and by examining the effects of site-selective antioxidants on cell survival after fenretinide treatment. Studies of mitochondrial electron transport employed specific inhibitors of individual proteins in the electron transport chain.
Results: Knockdown of p75NTR attenuates fenretinide-induced accumulation of mitochondrial superoxide and apoptosis. Overexpression of p75NTR has the opposite effects. Pretreatment of cells with 2-thenoyltrifluoroacetone or dehydroascorbic acid uniquely prevents mitochondrial superoxide accumulation and cell death after fenretinide treatment, indicating that mitochondrial complex II is the likely site of fenretinide-induced superoxide generation and p75NTR-induced potentiation of these phenomena.
Conclusion: Modification of expression of p75NTR in a particular neuroblastoma cell line modifies its susceptibility to fenretinide. Enhancers of p75NTR expression or signaling could be potential drugs for use as adjuncts to chemotherapy of neural tumors.
Conflict of interest statement
None of the authors have any real or perceivable conflicts of interest or financial relationships relevant to the contents of this manuscript.
Figures

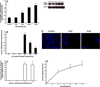

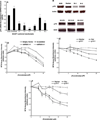
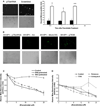
References
-
- Bartkowska K, Turlejski K, Djavadian RL. Neurotrophins and their receptors in early development of the mammalian nervous system. Acta Neurobiol Exp. 2010;70:454–467. - PubMed
-
- Yan C, Liang Y, Nylander KD, et al. p75-NGF as an antiapoptotic complex: independence vs. cooperativity. Mol Pharmacol. 2002;61:710–719. - PubMed
-
- Buhren J, Christoph AHA, Buslei R, et al. Expression of the neurotrophin receptor p75NTR in medulloblastomas is correlated with distinct histological and clinical features: evidence for a medulloblastoma subtype derived from the external granule cell layer. J Neuropathol Exp Neurol. 2000;59:229–240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

