A phase 2 study of KX2-391, an oral inhibitor of Src kinase and tubulin polymerization, in men with bone-metastatic castration-resistant prostate cancer
- PMID: 23314737
- PMCID: PMC3609871
- DOI: 10.1007/s00280-013-2079-z
A phase 2 study of KX2-391, an oral inhibitor of Src kinase and tubulin polymerization, in men with bone-metastatic castration-resistant prostate cancer
Abstract
Purpose: KX2-391 is an oral non-ATP-competitive inhibitor of Src kinase and tubulin polymerization. In phase 1 trials, prostate-specific antigen (PSA) declines were seen in patients with advanced prostate cancer. We conducted a single-arm phase 2 study evaluating KX2-391 in men with chemotherapy-naïve bone-metastatic castration-resistant prostate cancer (CRPC).
Methods: We treated 31 patients with oral KX2-391 (40 mg twice-daily) until disease progression or unacceptable toxicity. The primary endpoint was 24-week progression-free survival (PFS); a 50 % success rate was pre-defined as clinically significant. Secondary endpoints included PSA progression-free survival (PPFS) and PSA response rates. Exploratory outcomes included pharmacokinetic studies, circulating tumor cell (CTC) enumeration, and analysis of markers of bone resorption [urinary N-telopeptide (uNTx); C-telopeptide (CTx)] and formation [bone alkaline phosphatase (BAP); osteocalcin].
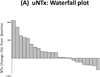
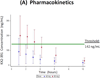
Results: The trial closed early after accrual of 31 patients, due to a pre-specified futility rule. PFS at 24 weeks was 8 %, and median PFS was 18.6 weeks. The PSA response rate (≥ 30 % decline) was 10 %, and median PPFS was 5.0 weeks. Additionally, 18 % of men with unfavorable (≥ 5) CTCs at baseline converted to favorable (<5) CTCs with treatment. The proportion of men with declines in bone turnover markers was 32 % for uNTx, 21 % for CTx, 10 % for BAP, and 25 % for osteocalcin. In pharmacokinetic studies, median C max was 61 (range 16-129) ng/mL, and median AUC was 156 (35-348) ng h/mL. Common toxicities included hepatic derangements, myelosuppression, fatigue, nausea, and constipation.
Conclusion: KX2-391 dosed at 40 mg twice-daily lacks antitumor activity in men with CRPC, but has modest effects on bone turnover markers. Because a C max of ≥142 ng/mL is required for tubulin polymerization inhibition (defined from preclinical studies), higher once-daily dosing will be used in future trials.
Conflict of interest statement
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

