The influence of therapeutic radiation on the patterns of bone remodeling in ovary-intact and ovariectomized mice
- PMID: 23314741
- PMCID: PMC3595353
- DOI: 10.1007/s00223-012-9688-0
The influence of therapeutic radiation on the patterns of bone remodeling in ovary-intact and ovariectomized mice
Abstract
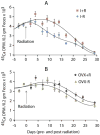
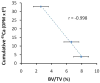
Our purpose was to characterize changes in bone remodeling associated with localized radiation that models therapeutic cancer treatment in ovary-intact (I) and ovariectomized (OVX) mice and to evaluate the influence of radiation on the pattern of bone mineral remodeling. Young adult, female BALB/c mice, I and OVX, were used (n = 71). All mice were intravenously injected with 15 μCi (45)Ca. Thirty days post-(45)Ca administration, the hind limbs of 17 mice were exposed to a single dose of 16 Gy radiation (R). The time course of (45)Ca excretion, serum CTx and osteocalcin markers, and cancellous bone volume fraction (BV/TV) and cortical thickness (Ct.Th) of the distal femur were assayed. Cellular activity and dynamic histomorphometry were performed. Irradiation resulted in rapid increases in fecal (45)Ca excretion compared to control groups, indicating increased bone remodeling. CTx increased rapidly after irradiation, followed by an increase in osteocalcin concentration. BV/TV decreased in the I mice following irradiation. Ct.Th increased in the OVX groups following irradiation. I+R mice exhibited diminished osteoblast surface, osteoclast number, and mineral apposition. Our murine model showed the systemic effects (via (45)Ca excretion) and local effects (via bone microarchitecture and surface activity) of clinically relevant, therapeutic radiation exposure. The I and OVX murine models have similar (45)Ca excretion but different bone microarchitectural responses. The (45)Ca assay effectively indicates the onset and rate of systemic bone mineral remodeling, providing real-time assessment of changes in bone histomorphometric parameters. Monitoring bone health via a bone mineral marker may help to identify the appropriate time for clinical intervention to preserve skeletal integrity.
Conflict of interest statement
The authors have stated that they have no conflict of interest.
Figures


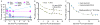
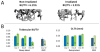
References
-
- Guise T. Bone loss and fracture risk associated with cancer therapy. The Oncologist. 2006;11:1121–1131. - PubMed
-
- Chen Z, Maricic M, Bassford T, Pettinger M, Ritenbaugh C, Lopez A, Barad D, Gass M, LeBoff M. Fracture risk among breast cancer survivors: Results from the Women’s Health Initiative Observational Study. Archives of Internal Medicine. 2005;165:552. - PubMed
-
- Baxter N, Habermann E, Tepper J, Durham S, Virnig B. Risk of pelvic fractures in older women following pelvic irradiation. Am Med Assoc. 2005:2587–2593. - PubMed
-
- Garnero P. Markers of bone turnover for the prediction of fracture risk. Osteoporos Int. 2000;11(Suppl 6):S55–65. - PubMed
-
- Johnell O, Oden A, De Laet C, Garnero P, Delmas P, Kanis J. Biochemical indices of bone turnover and the assessment of fracture probability. Osteoporosis International. 2002;13:523–526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

