ER stress signalling through eIF2α and CHOP, but not IRE1α, attenuates adipogenesis in mice
- PMID: 23314846
- PMCID: PMC3606029
- DOI: 10.1007/s00125-012-2809-5
ER stress signalling through eIF2α and CHOP, but not IRE1α, attenuates adipogenesis in mice
Abstract
Aims/hypothesis: Although obesity is associated with endoplasmic reticulum (ER) stress and activation of the unfolded protein response (UPR) in adipose tissue, it is not known how UPR signalling affects adipogenesis. To test whether signalling through protein kinase RNA-like ER kinase/eukaryotic initiation factor 2 alpha (PERK/eIF2α) or inositol-requiring enzyme 1 alpha/X-box binding protein 1 (IRE1α/XBP1) is required for adipogenesis, we studied the role of UPR signalling in adipocyte differentiation in vitro and in vivo in mice.
Methods: The role of UPR signalling in adipogenesis was investigated using 3T3-L1 cells and primary mouse embryonic fibroblasts (MEFs) by activation or inhibition of PERK-mediated phosphorylation of the eIF2α- and IRE1α-mediated splicing of Xbp1 mRNA. Body weight change, fat mass composition and adipocyte number and size were measured in wild-type and genetically engineered mice fed a control or high-fat diet (HFD).
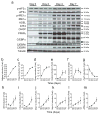
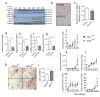
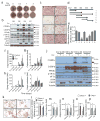
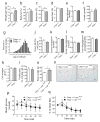
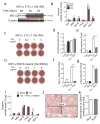
Results: ER stress repressed adipocyte differentiation in 3T3-L1 cells. Impaired eIF2α phosphorylation enhanced adipocyte differentiation in MEFs, as well as in mice. In contrast, increased eIF2α phosphorylation reduced adipocyte differentiation in 3T3-L1 cells. Forced production of CCAAT/enhancer binding protein (C/EBP) homologous protein (CHOP), a downstream target of eIF2α phosphorylation, inhibited adipogenesis in 3T3-L1 cells. Mice with deletion of Chop (also known as Ddit3) (Chop (-/-)) gained more fat mass than wild-type mice on HFD. In addition, Chop deletion in genetically obese Lepr (db/db) mice increased body fat mass without altering adipocyte size. In contrast to the eIF2α-CHOP pathway, activation or deletion of Ire1a (also known as Ern1) did not alter adipocyte differentiation in 3T3-L1 cells.
Conclusions/interpretation: These results demonstrate that eIF2α-CHOP suppresses adipogenesis and limits expansion of fat mass in vivo in mice, rendering this pathway a potential therapeutic target.
Conflict of interest statement
The authors declare that there is no duality of interest associated with this manuscript.
Figures


References
-
- Barness LA, Opitz JM, Gilbert-Barness E. Obesity: genetic, molecular, and environmental aspects. Am J Med Genet A. 2007;143A:3016–3034. - PubMed
-
- Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiology & Behavior. 2008;94:206–218. - PubMed
-
- de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008;54:945–955. - PubMed
-
- Faust I, Johnson P, Stern J, Hirsch J. Diet-induced adipocyte number increase in adult rats: a new model of obesity. Am J Physiol. 1978;235:E279–E286. - PubMed
-
- Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK088227/DK/NIDDK NIH HHS/United States
- DK042394/DK/NIDDK NIH HHS/United States
- DK093074/DK/NIDDK NIH HHS/United States
- R24 DK093074/DK/NIDDK NIH HHS/United States
- HL052173/HL/NHLBI NIH HHS/United States
- R37 DK042394/DK/NIDDK NIH HHS/United States
- P60 DK020572/DK/NIDDK NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- K08 DK097178/DK/NIDDK NIH HHS/United States
- P01 HL057346/HL/NHLBI NIH HHS/United States
- DK088227/DK/NIDDK NIH HHS/United States
- R01 DK042394/DK/NIDDK NIH HHS/United States
- R01 HL052173/HL/NHLBI NIH HHS/United States
- DK020572/DK/NIDDK NIH HHS/United States
- HL057346/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

