Aldosterone deficiency prevents high-fat-feeding-induced hyperglycaemia and adipocyte dysfunction in mice
- PMID: 23314847
- PMCID: PMC3593801
- DOI: 10.1007/s00125-012-2814-8
Aldosterone deficiency prevents high-fat-feeding-induced hyperglycaemia and adipocyte dysfunction in mice
Abstract
Aims/hypothesis: Obesity is associated with aldosterone excess, hypertension and the metabolic syndrome, but the relative contribution of aldosterone to obesity-related complications is debated. We previously demonstrated that aldosterone impairs insulin secretion, and that genetic aldosterone deficiency increases glucose-stimulated insulin secretion in vivo. We hypothesised that elimination of endogenous aldosterone would prevent obesity-induced insulin resistance and hyperglycaemia.
Methods: Wild-type and aldosterone synthase-deficient (As (-/-)) mice were fed a high-fat (HF) or normal chow diet for 12 weeks. We assessed insulin sensitivity and insulin secretion using clamp methodology and circulating plasma adipokines, and examined adipose tissue via histology.
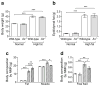
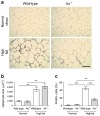
Results: HF diet induced weight gain similarly in the two groups, but As (-/-) mice were protected from blood glucose elevation. HF diet impaired insulin sensitivity similarly in As (-/-) and wild-type mice, assessed by hyperinsulinaemic-euglycaemic clamps. Fasting and glucose-stimulated insulin were higher in HF-fed As (-/-) mice than in wild-type controls. Although there was no difference in insulin sensitivity during HF feeding in As (-/-) mice compared with wild-type controls, fat mass, adipocyte size and adiponectin increased, while adipose macrophage infiltration decreased. HF feeding significantly increased hepatic steatosis and triacylglycerol content in wild-type mice, which was attenuated in aldosterone-deficient mice.
Conclusions/interpretation: These studies demonstrate that obesity induces insulin resistance independently of aldosterone and adipose tissue inflammation, and suggest a novel role for aldosterone in promoting obesity-induced beta cell dysfunction, hepatic steatosis and adipose tissue inflammation.
Conflict of interest statement
Figures
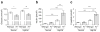




References
-
- Goodfriend TL, Kelley DE, Goodpaster BH, Winters SJ. Visceral obesity and insulin resistance are associated with plasma aldosterone levels in women. Obes Res. 1999;7:355–362. - PubMed
-
- Vasan RS, Evans JC, Larson MG, et al. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med. 2004;351:33–41. - PubMed
-
- Ingelsson E, Pencina MJ, Tofler GH, et al. Multimarker approach to evaluate the incidence of the metabolic syndrome and longitudinal changes in metabolic risk factors: the Framingham Offspring Study. Circulation. 2007;116:984–992. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK038226-24/DK/NIDDK NIH HHS/United States
- DK58404/DK/NIDDK NIH HHS/United States
- R01 DK096994/DK/NIDDK NIH HHS/United States
- K23 DK081662/DK/NIDDK NIH HHS/United States
- DK20593/DK/NIDDK NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- DK081662/DK/NIDDK NIH HHS/United States
- P60 DK020593/DK/NIDDK NIH HHS/United States
- R01 HL060906/HL/NHLBI NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- P01 DK038226/DK/NIDDK NIH HHS/United States
- R01 DK095761/DK/NIDDK NIH HHS/United States
- HL060906/HL/NHLBI NIH HHS/United States
- DK59637/DK/NIDDK NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

