Self double-stranded (ds)DNA induces IL-1β production from human monocytes by activating NLRP3 inflammasome in the presence of anti-dsDNA antibodies
- PMID: 23315075
- PMCID: PMC3563755
- DOI: 10.4049/jimmunol.1201195
Self double-stranded (ds)DNA induces IL-1β production from human monocytes by activating NLRP3 inflammasome in the presence of anti-dsDNA antibodies
Abstract
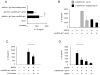
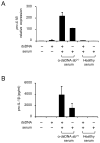
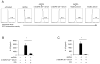
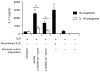
The pathogenic hallmark of systemic lupus erythematosus is the autoimmune response against self nuclear Ags, including dsDNA. The increased expression of the proinflammatory cytokine IL-1β has been found in the cutaneous lesion and PBMCs from lupus patients, suggesting a potential involvement of this cytokine in the pathogenesis of lupus. IL-1β is produced primarily by innate immune cells such as monocytes and can promote a Th17 cell response, which is increased in lupus. IL-1β production requires cleaving pro-IL-β into IL-1β by the caspase-1-associated multiprotein complex called inflammasomes. In this study we show that self dsDNA induces IL-1β production from human monocytes dependent on serum or purified IgG containing anti-dsDNA Abs by activating the nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome. Reactive oxygen species (ROS) and K(+) efflux were involved in this activation. Knocking down the NLRP3 or inhibiting caspase-1, ROS, and K(+) efflux decreased IL-1β production. Supernatants from monocytes treated with a combination of self dsDNA and anti-dsDNA Ab(+) serum promoted IL-17 production from CD4(+) T cells in an IL-1β-dependent manner. These findings provide new insights in lupus pathogenesis by demonstrating that self dsDNA together with its autoantibodies induces IL-1β production from human monocytes by activating the NLRP3 inflammasome through inducing ROS synthesis and K(+) efflux, leading to the increased Th17 cell response.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Anti-dsDNA antibodies bind to TLR4 and activate NLRP3 inflammasome in lupus monocytes/macrophages.J Transl Med. 2016 Jun 1;14(1):156. doi: 10.1186/s12967-016-0911-z. J Transl Med. 2016. PMID: 27250627 Free PMC article.
-
U1-small nuclear ribonucleoprotein activates the NLRP3 inflammasome in human monocytes.J Immunol. 2012 May 15;188(10):4769-75. doi: 10.4049/jimmunol.1103355. Epub 2012 Apr 6. J Immunol. 2012. PMID: 22490866 Free PMC article.
-
The anti-tumorigenic mushroom Agaricus blazei Murill enhances IL-1β production and activates the NLRP3 inflammasome in human macrophages.PLoS One. 2012;7(7):e41383. doi: 10.1371/journal.pone.0041383. Epub 2012 Jul 23. PLoS One. 2012. PMID: 22844468 Free PMC article.
-
Helicobacter pylori induces IL-1β and IL-18 production in human monocytic cell line through activation of NLRP3 inflammasome via ROS signaling pathway.Pathog Dis. 2015 Jun;73(4):ftu024. doi: 10.1093/femspd/ftu024. Epub 2015 Jan 5. Pathog Dis. 2015. PMID: 25834143
-
The Role of Inflammasomes in Glomerulonephritis.Int J Mol Sci. 2022 Apr 11;23(8):4208. doi: 10.3390/ijms23084208. Int J Mol Sci. 2022. PMID: 35457026 Free PMC article. Review.
Cited by
-
Targeting epigenetic regulators for inflammation: Mechanisms and intervention therapy.MedComm (2020). 2022 Sep 15;3(4):e173. doi: 10.1002/mco2.173. eCollection 2022 Dec. MedComm (2020). 2022. PMID: 36176733 Free PMC article. Review.
-
Programmed Cell Death Pathways in the Pathogenesis of Systemic Lupus Erythematosus.J Immunol Res. 2019 Dec 1;2019:3638562. doi: 10.1155/2019/3638562. eCollection 2019. J Immunol Res. 2019. PMID: 31871956 Free PMC article. Review.
-
Cytosolic Internalization of Anti-DNA Antibodies by Human Monocytes Induces Production of Pro-inflammatory Cytokines Independently of the Tripartite Motif-Containing 21 (TRIM21)-Mediated Pathway.Front Immunol. 2018 Sep 4;9:2019. doi: 10.3389/fimmu.2018.02019. eCollection 2018. Front Immunol. 2018. PMID: 30233598 Free PMC article.
-
An essential role of caspase 1 in the induction of murine lupus and its associated vascular damage.Arthritis Rheumatol. 2014 Jan;66(1):152-62. doi: 10.1002/art.38225. Arthritis Rheumatol. 2014. PMID: 24449582 Free PMC article.
-
Lactoferrin-Containing Immunocomplexes Drive the Conversion of Human Macrophages from M2- into M1-like Phenotype.Front Immunol. 2018 Jan 23;9:37. doi: 10.3389/fimmu.2018.00037. eCollection 2018. Front Immunol. 2018. PMID: 29410669 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

