EBV BILF1 evolved to downregulate cell surface display of a wide range of HLA class I molecules through their cytoplasmic tail
- PMID: 23315076
- PMCID: PMC3565383
- DOI: 10.4049/jimmunol.1102462
EBV BILF1 evolved to downregulate cell surface display of a wide range of HLA class I molecules through their cytoplasmic tail
Abstract
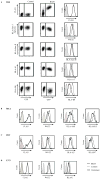
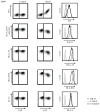
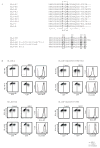
Coevolution of herpesviruses and their hosts has driven the development of both host antiviral mechanisms to detect and eliminate infected cells and viral ploys to escape immune surveillance. Among the immune-evasion strategies used by the lymphocryptovirus (γ(1)-herpesvirus) EBV is the downregulation of surface HLA class I expression by the virally encoded G protein-coupled receptor BILF1, thereby impeding presentation of viral Ags and cytotoxic T cell recognition of the infected cell. In this study, we show EBV BILF1 to be expressed early in the viral lytic cycle. BILF1 targets a broad range of HLA class I molecules, including multiple HLA-A and -B types and HLA-E. In contrast, HLA-C was only marginally affected. We advance the mechanistic understanding of the process by showing that the cytoplasmic C-terminal tail of EBV BILF1 is required for reducing surface HLA class I expression. Susceptibility to BILF1-mediated downregulation, in turn, is conferred by specific residues in the intracellular tail of the HLA class I H chain. Finally, we explore the evolution of BILF1 within the lymphocryptovirus genus. Although the homolog of BILF1 encoded by the lymphocryptovirus infecting Old World rhesus primates shares the ability of EBV to downregulate cell surface HLA class I expression, this function is not possessed by New World marmoset lymphocryptovirus BILF1. Therefore, this study furthers our knowledge of the evolution of immunoevasive functions by the lymphocryptovirus genus of herpesviruses.
Figures

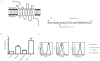


References
-
- Kieff E, Rickinson AB. Epstein-Barr Virus and Its Replication. In: Knipe DM, Howley PM, editors. Field’s Virology. Lippincott Williams & Wilkins; Philadelphia: 2007. p. 2603.
-
- Rickinson AB, Kieff E. Epstein-Barr Virus. In: Knipe DM, Howley PM, editors. Field’s Virology. Vol. 2. Lippincott Williams & Wilkins; Philadelphia: 2007. p. 2655.
-
- Kutok JL, Wang F. Spectrum of Epstein-Barr virus-associated diseases. Annu Rev Pathol. 2006;1:375–404. - PubMed
-
- Hislop AD, Taylor GS, Sauce D, Rickinson AB. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu Rev Immunol. 2007;25:587–617. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

