T cells home to the thymus and control infection
- PMID: 23315077
- PMCID: PMC3563877
- DOI: 10.4049/jimmunol.1202412
T cells home to the thymus and control infection
Abstract
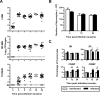
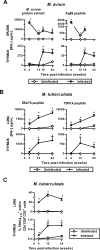
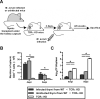
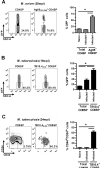
The thymus is a target of multiple pathogens. How the immune system responds to thymic infection is largely unknown. Despite being considered an immune-privileged organ, we detect a mycobacteria-specific T cell response in the thymus following dissemination of Mycobacterium avium or Mycobacterium tuberculosis. This response includes proinflammatory cytokine production by mycobacteria-specific CD4(+) and CD8(+) T cells, which stimulates infected cells and controls bacterial growth in the thymus. Importantly, the responding T cells are mature peripheral T cells that recirculate back to the thymus. The recruitment of these cells is associated with an increased expression of Th1 chemokines and an enrichment of CXCR3(+) mycobacteria-specific T cells in the thymus. Finally, we demonstrate it is the mature T cells that home to the thymus that most efficiently control mycobacterial infection. Although the presence of mature T cells in the thymus has been recognized for some time, to our knowledge, these data are the first to show that T cell recirculation from the periphery to the thymus is a mechanism that allows the immune system to respond to thymic infection. Maintaining a functional thymic environment is essential to maintain T cell differentiation and prevent the emergence of central tolerance to the invading pathogens.
Figures




Comment in
-
Mature peripheral T cells are important to preserve thymus function and selection of thymocytes during Mycobacterium tuberculosis infection.Immunotherapy. 2013 Jun;5(6):573-6. doi: 10.2217/imt.13.41. Immunotherapy. 2013. PMID: 23725281
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

