Patterns and causes of suboptimal response to tenofovir-based therapy in individuals coinfected with HIV and hepatitis B virus
- PMID: 23315316
- PMCID: PMC3693490
- DOI: 10.1093/cid/cit002
Patterns and causes of suboptimal response to tenofovir-based therapy in individuals coinfected with HIV and hepatitis B virus
Abstract
Background: Tenofovir (TDF) is effective for treatment of hepatitis B virus (HBV) in human immunodeficiency virus (HIV) infection; however, some individuals have ongoing HBV viremia, the reasons for which are unclear. We determined the patterns and factors associated with detectable HBV DNA in HIV-HBV-coinfected subjects on highly active antiretroviral therapy (HAART).
Methods: One hundred sixty-five HIV-HBV-coinfected individuals from the United States, Australia, and Thailand, the majority of whom were on HAART at study entry, were prospectively followed semiannually for a median of 2.8 years. Logistic regression was used to determine factors associated with detectable HBV DNA.
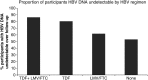
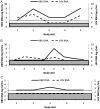
Results: Anti-HBV regimens were TDF/emtricitabine (57%), lamivudine or emtricitabine (19%), or TDF monotherapy (13%). During follow-up, HBV DNA was detected at 21% of study visits and was independently associated with hepatitis B e antigen (HBeAg), HAART <2 years, CD4 <200 cells/mm(3), detectable HIV RNA, reporting <95% adherence, and anti-HBV regimen. TDF/emtricitabine was less likely to be associated with detectable HBV than other regimens, including TDF monotherapy (odds ratio, 2.79; P = .02). In subjects on optimal anti-HBV therapy (TDF/emtricitabine) and with undetectable HIV RNA, HBeAg, CD4 <200 mm(3), and reporting <95% adherence remained associated with detectable HBV DNA. Three main patterns of HBV viremia were observed: persistent HBV viremia, viral rebound (>1 log from nadir), and viral blips. No TDF resistance was identified.
Conclusions: Tenofovir/emtricitabine was superior to other anti-HBV regimens in long-term HBV suppression. HBV viremia on therapy was identified in 1 of 3 main patterns. Suboptimal adherence was associated with detectable HBV DNA during therapy, even when HIV was undetectable.
Figures
References
-
- Hoffmann CJ, Thio CL. Clinical implications of HIV and hepatitis B co-infection in Asia and Africa. Lancet Infect Dis. 2007;7:402–9. - PubMed
-
- Matthews G, Cooper DA, Dore G. Improvements in parameters of end stage liver disease in patients with HIV/HBV-related cirrhosis treated with tenofovir. Antiviral Therapy. 2007;12:119–22. - PubMed
-
- Benhamou Y, Fleury H, Trimoulet P, et al. Anti-hepatitis B virus efficacy of tenofovir disoproxil fumarate in HIV-infected patients. Hepatology. 2006;43:548–55. - PubMed
-
- Nelson M, Portsmouth S, Stebbing J, et al. An open-label study of tenofovir in HIV-1 and hepatitis B virus coinfected individuals. AIDS. 2003;17:F7–10. - PubMed
-
- Martin-Carbonero L, Teixeira T, Poveda E, et al. Clinical and virological outcomes in HIV-infected patients with chronic hepatitis B on long-term nucleos(t)ide analogues. AIDS. 2011;25:73–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 AI035042/AI/NIAID NIH HHS/United States
- UL1 RR025005/RR/NCRR NIH HHS/United States
- R56 AI060449/AI/NIAID NIH HHS/United States
- UO1-AI-35041/AI/NIAID NIH HHS/United States
- U01 AI035041/AI/NIAID NIH HHS/United States
- UL1-RR025005/RR/NCRR NIH HHS/United States
- U01 AI035043/AI/NIAID NIH HHS/United States
- UO1-AI-35039/AI/NIAID NIH HHS/United States
- U01 AI035040/AI/NIAID NIH HHS/United States
- U01 AI035039/AI/NIAID NIH HHS/United States
- UO1-AI-35043/AI/NIAID NIH HHS/United States
- UO1-AI-35040/AI/NIAID NIH HHS/United States
- R56AI60449/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

