Trends and disparities in antiretroviral therapy initiation and virologic suppression among newly treatment-eligible HIV-infected individuals in North America, 2001-2009
- PMID: 23315317
- PMCID: PMC3657490
- DOI: 10.1093/cid/cit003
Trends and disparities in antiretroviral therapy initiation and virologic suppression among newly treatment-eligible HIV-infected individuals in North America, 2001-2009
Abstract
Background: Since the mid-1990s, effective antiretroviral therapy (ART) regimens have improved in potency, tolerability, ease of use, and class diversity. We sought to examine trends in treatment initiation and resulting human immunodeficiency virus (HIV) virologic suppression in North America between 2001 and 2009, and demographic and geographic disparities in these outcomes.
Methods: We analyzed data on HIV-infected individuals newly clinically eligible for ART (ie, first reported CD4+ count<350 cells/µL or AIDS-defining illness, based on treatment guidelines during the study period) from 17 North American AIDS Cohort Collaboration on Research and Design cohorts. Outcomes included timely ART initiation (within 6 months of eligibility) and virologic suppression (≤500 copies/mL, within 1 year). We examined time trends and considered differences by geographic location, age, sex, transmission risk, race/ethnicity, CD4+ count, and viral load, and documented psychosocial barriers to ART initiation, including non-injection drug abuse, alcohol abuse, and mental illness.
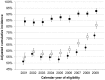
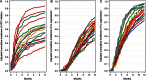
Results: Among 10,692 HIV-infected individuals, the cumulative incidence of 6-month ART initiation increased from 51% in 2001 to 72% in 2009 (Ptrend<.001). The cumulative incidence of 1-year virologic suppression increased from 55% to 81%, and among ART initiators, from 84% to 93% (both Ptrend<.001). A greater number of psychosocial barriers were associated with decreased ART initiation, but not virologic suppression once ART was initiated. We found significant heterogeneity by state or province of residence (P<.001).
Conclusions: In the last decade, timely ART initiation and virologic suppression have greatly improved in North America concurrent with the development of better-tolerated and more potent regimens, but significant barriers to treatment uptake remain, both at the individual level and systemwide.
Figures
References
-
- Boyd MA. Improvements in antiretroviral therapy outcomes over calendar time. Curr Opin HIV AIDS. 2009;4:194–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01-AI35043/AI/NIAID NIH HHS/United States
- K24 DA000432/DA/NIDA NIH HHS/United States
- K24-DA00432/DA/NIDA NIH HHS/United States
- P30-AI27757/AI/NIAID NIH HHS/United States
- P30 AI036219/AI/NIAID NIH HHS/United States
- R01 AA016893/AA/NIAAA NIH HHS/United States
- U01-AI34989/AI/NIAID NIH HHS/United States
- P30 AI027767/AI/NIAID NIH HHS/United States
- U01 AI035042/AI/NIAID NIH HHS/United States
- U01 DA036297/DA/NIDA NIH HHS/United States
- F31 DA030254/DA/NIDA NIH HHS/United States
- CBR-86906/CAPMC/ CIHR/Canada
- R01-AA16893/AA/NIAAA NIH HHS/United States
- M01 RR000079/RR/NCRR NIH HHS/United States
- R24-AI067039/AI/NIAID NIH HHS/United States
- U01 AI037984/AI/NIAID NIH HHS/United States
- U01-HD32632/HD/NICHD NIH HHS/United States
- R01 DA011602/DA/NIDA NIH HHS/United States
- K01 AI071725/AI/NIAID NIH HHS/United States
- P30-AI27763/AI/NIAID NIH HHS/United States
- U01-AI34993/AI/NIAID NIH HHS/United States
- M01-RR00083/RR/NCRR NIH HHS/United States
- TGF-96118/CAPMC/ CIHR/Canada
- U01 AI031834/AI/NIAID NIH HHS/United States
- HCP-97105/CAPMC/ CIHR/Canada
- K01-AI071754/AI/NIAID NIH HHS/United States
- P30 AI094189/AI/NIAID NIH HHS/United States
- M01-RR00079/RR/NCRR NIH HHS/United States
- U01 AI035004/AI/NIAID NIH HHS/United States
- G12 MD007583/MD/NIMHD NIH HHS/United States
- P30 AI054999/AI/NIAID NIH HHS/United States
- R01-DA04334/DA/NIDA NIH HHS/United States
- U01-AI35040/AI/NIAID NIH HHS/United States
- R01 DA004334/DA/NIDA NIH HHS/United States
- U01-AI37984/AI/NIAID NIH HHS/United States
- M01-RR00071/RR/NCRR NIH HHS/United States
- U01-AI35004/AI/NIAID NIH HHS/United States
- CDC200-2006-18797/PHS HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- P30-AI036219/AI/NIAID NIH HHS/United States
- U01 AI034989/AI/NIAID NIH HHS/United States
- U01 AI037613/AI/NIAID NIH HHS/United States
- KRS-86251/CAPMC/ CIHR/Canada
- M01 RR000071/RR/NCRR NIH HHS/United States
- AHQ290-01-0012/PHS HHS/United States
- U01-AI35042/AI/NIAID NIH HHS/United States
- M01 RR000722/RR/NCRR NIH HHS/United States
- M01-RR025747/RR/NCRR NIH HHS/United States
- CBR-94036/CAPMC/ CIHR/Canada
- U01 AI035041/AI/NIAID NIH HHS/United States
- R24 AI067039/AI/NIAID NIH HHS/United States
- U01-AI37613/AI/NIAID NIH HHS/United States
- M01-RR-00052/RR/NCRR NIH HHS/United States
- U01-AI069918/AI/NIAID NIH HHS/United States
- K24-AI1065298/AI/NIAID NIH HHS/United States
- U01-AI42590/AI/NIAID NIH HHS/United States
- K01 AI071754/AI/NIAID NIH HHS/United States
- P30-AI50410/AI/NIAID NIH HHS/United States
- UL1-RR024131/RR/NCRR NIH HHS/United States
- R01-DA12568/DA/NIDA NIH HHS/United States
- U01-AI31834/AI/NIAID NIH HHS/United States
- U01 AI034994/AI/NIAID NIH HHS/United States
- M01 RR000052/RR/NCRR NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- K01 AI093197/AI/NIAID NIH HHS/United States
- U01 AI069918/AI/NIAID NIH HHS/United States
- U01-AI35041/AI/NIAID NIH HHS/United States
- K23 DA019809/DA/NIDA NIH HHS/United States
- N02 CP055504/CP/NCI NIH HHS/United States
- U01 AI035043/AI/NIAID NIH HHS/United States
- K23-AI610320/AI/NIAID NIH HHS/United States
- P30 AI027757/AI/NIAID NIH HHS/United States
- UL1 RR025747/RR/NCRR NIH HHS/United States
- U01-AI35039/AI/NIAID NIH HHS/United States
- R01 DA012568/DA/NIDA NIH HHS/United States
- F31-DA30254/DA/NIDA NIH HHS/United States
- P30-AI27767/AI/NIAID NIH HHS/United States
- U01 AI035040/AI/NIAID NIH HHS/United States
- K01-AI093197/AI/NIAID NIH HHS/United States
- U01 AI034993/AI/NIAID NIH HHS/United States
- R01-DA11602/DA/NIDA NIH HHS/United States
- M01 RR000083/RR/NCRR NIH HHS/United States
- K01-AI071725/AI/NIAID NIH HHS/United States
- U01-AI34994/AI/NIAID NIH HHS/United States
- 169621/CAPMC/ CIHR/Canada
- U01 AI035039/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- U01 HD032632/HD/NICHD NIH HHS/United States
- U01 AI042590/AI/NIAID NIH HHS/United States
- P30-AI54999/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

