Prospective, multicenter study of ventricular assist device infections
- PMID: 23315371
- PMCID: PMC3695607
- DOI: 10.1161/CIRCULATIONAHA.112.128132
Prospective, multicenter study of ventricular assist device infections
Abstract
Background: Ventricular assist devices (VADs) improve survival and quality of life in patients with advanced heart failure, but their use is frequently complicated by infection. There are limited data on the microbiology and epidemiology of these infections.
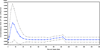
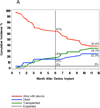
Methods and results: One hundred fifty patients scheduled for VAD implantation were enrolled (2006-2008) at 11 US cardiac centers and followed prospectively until transplantation, explantation for recovery, death, or for 1 year. Eighty-six patients (57%) received HeartMate II devices. Data were collected on potential preoperative, intraoperative, and postoperative risk factors for infection. Clinical, laboratory, and microbiological data were collected for suspected infections and evaluated by an infectious diseases specialist. Thirty-three patients (22%) developed 34 VAD-related infections with an incidence rate of 0.10 per 100 person-days (95% confidence interval, 0.073-0.142). The median time to infection was 68 days. The driveline was the most commonly infected site (n=28); 18 (64%) were associated with invasive disease. Staphylococci were the most common pathogen (47%), but pseudomonas or other Gram-negative bacteria caused 32% of infections. A history of depression and elevated baseline serum creatinine were independent predictors of VAD infection (adjusted hazard ratio=2.8 [P=0.007] and 1.7 [P=0.023], respectively). The HeartMate II was not associated with a decreased risk of infection. VAD infection increased 1-year mortality (adjusted hazard ratio=5.6; P<0.0001).
Conclusions: This prospective, multicenter study demonstrates that infection frequently complicates VAD placement and is a continuing problem despite the use of newer, smaller devices. Depression and renal dysfunction may increase the risk of VAD infection. VAD infection is a serious consequence because it adversely affects patient survival.
Clinical trial registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT01471795.
Figures




References
-
- Frazier OH, Rose EA, Oz MC, Dembitsky W, McCarthy P, Radovancevic B, Poirier VL, Dasse KA. Multicenter clinical evaluation of the heartmate vented electric left ventricular assist system in patients awaiting heart transplantation. J Thorac Cardiovasc Surg. 2001;122:1186–1195. - PubMed
-
- Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, Watson JT, Meier P, Ronan NS, Shapiro PA, Lazar RM, Miller LW, Gupta L, Frazier OH, Desvigne-Nickens P, Oz MC, Poirier VL. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med. 2001;345:1435–1443. - PubMed
-
- Holman WL, Park SJ, Long JW, Weinberg A, Gupta L, Tierney AR, Adamson RM, Watson JD, Raines EP, Couper GS, Pagani FD, Burton NA, Miller LW, Naka Y. Infection in permanent circulatory support: Experience from the rematch trial. J Heart Lung Transplant. 2004;23:1359–1365. - PubMed
-
- Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, Conte JV, Naka Y, Mancini D, Delgado RM, MacGillivray TE, Farrar DJ, Frazier OH. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

