How long has NICE taken to produce Technology Appraisal guidance? A retrospective study to estimate predictors of time to guidance
- PMID: 23315516
- PMCID: PMC3549260
- DOI: 10.1136/bmjopen-2012-001870
How long has NICE taken to produce Technology Appraisal guidance? A retrospective study to estimate predictors of time to guidance
Abstract
Objectives: To assess how long the UK's National Institute for Health and Clinical Excellence's (NICE) Technology Appraisal Programme has taken to produce guidance and to determine independent predictors of time to guidance.
Design: Retrospective time to event (survival) analysis.
Setting: Technology Appraisal guidance produced by NICE. DATASOURCE: All appraisals referred to NICE by February 2010 were included, except those referred prior to 2001 and a number that were suspended.
Outcome measure: Duration from the start of an appraisal (when the scope document was released) until publication of guidance.
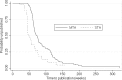
Results: Single Technology Appraisals (STAs) were published significantly faster than Multiple Technology Appraisals (MTAs) with median durations of 48.0 (IQR; 44.3-75.4) and 74.0 (IQR; 60.9-114.0) weeks, respectively (p <0.0001). Median time to publication exceeded published process timelines, even after adjusting for appeals. Results from the modelling suggest that STAs published guidance significantly faster than MTAs after adjusting for other covariates (by 36.2 weeks (95% CI -46.05 to -26.42 weeks)) and that appeals against provisional guidance significantly increased the time to publication (by 42.83 weeks (95% CI 35.50 to 50.17 weeks)). There was no evidence that STAs of cancer-related technologies took longer to complete compared with STAs of other technologies after adjusting for potentially confounding variables and only weak evidence suggesting that the time to produce guidance is increasing each year (by 1.40 weeks (95% CI -0.35 to 2.94 weeks)).
Conclusions: The results from this study suggest that the STA process has resulted in significantly faster guidance compared with the MTA process irrespective of the topic, but that these gains are lost if appeals are made against provisional guidance. While NICE processes continue to evolve over time, a trade-off might be that decisions take longer but at present there is no evidence of a significant increase in duration.
Figures
Similar articles
-
Single technology appraisals by NICE: are they delivering faster guidance to the NHS?Pharmacoeconomics. 2008;26(12):1037-43. doi: 10.2165/0019053-200826120-00006. Pharmacoeconomics. 2008. PMID: 19014204
-
The National Institute for Health and Clinical Excellence Single Technology Appraisal process: lessons from the first 4 years.Value Health. 2011 Dec;14(8):1158-65. doi: 10.1016/j.jval.2011.06.007. Epub 2011 Sep 8. Value Health. 2011. PMID: 22152188
-
NICE guidance: a comparative study of the introduction of the single technology appraisal process and comparison with guidance from Scottish Medicines Consortium.BMJ Open. 2012 Jan 30;2(1):e000671. doi: 10.1136/bmjopen-2011-000671. Print 2012. BMJ Open. 2012. PMID: 22290398 Free PMC article.
-
Does Methodological Guidance Produce Consistency? A Review of Methodological Consistency in Breast Cancer Utility Value Measurement in NICE Single Technology Appraisals.Pharmacoecon Open. 2018 Jun;2(2):97-107. doi: 10.1007/s41669-017-0040-5. Pharmacoecon Open. 2018. PMID: 29623616 Free PMC article. Review.
-
Modelling approaches for histology-independent cancer drugs to inform NICE appraisals: a systematic review and decision-framework.Health Technol Assess. 2021 Dec;25(76):1-228. doi: 10.3310/hta25760. Health Technol Assess. 2021. PMID: 34990339
Cited by
-
Health Technology Assessment of Drugs in Ireland: An Analysis of Timelines.Pharmacoecon Open. 2020 Jun;4(2):287-296. doi: 10.1007/s41669-019-00177-8. Pharmacoecon Open. 2020. PMID: 31531843 Free PMC article.
-
NICE and Fair? Health Technology Assessment Policy Under the UK's National Institute for Health and Care Excellence, 1999-2018.Health Care Anal. 2020 Sep;28(3):193-227. doi: 10.1007/s10728-019-00381-x. Health Care Anal. 2020. PMID: 31325000 Free PMC article.
-
New Medicines in Wales: The All Wales Medicines Strategy Group (AWMSG) Appraisal Process and Outcomes.Pharmacoeconomics. 2018 May;36(5):613-624. doi: 10.1007/s40273-018-0632-7. Pharmacoeconomics. 2018. PMID: 29520603 Free PMC article.
-
Barriers and facilitators to the implementation of guidelines in rare diseases: a systematic review.Orphanet J Rare Dis. 2023 Jun 7;18(1):140. doi: 10.1186/s13023-023-02667-9. Orphanet J Rare Dis. 2023. PMID: 37286999 Free PMC article.
References
-
- National Institute for Health and Clinical Excellence Guide to the multiple technology appraisal process. London: National Institute for Health and Clinical Excellence, 2009
-
- Mayor S. NICE to issue faster guidance to the NHS. BMJ 2005;331:1101. - PubMed
-
- Haycox A. Does ‘NICE blight’ exist, and if so, why?. Pharmacoeconomics 2008;26:987–9 - PubMed
-
- NICE Guide to the single technology appraisal process. London: National Institute for Health and Clinical Excellence, 2009
-
- Wailoo A, Pickstone C. A review of the NICE Single Technology Appraisal process. Sheffield: School of Health and Related Research, University of Sheffield (Decision Support Unit), 2008
LinkOut - more resources
Full Text Sources
Other Literature Sources