Caffeate respiration in the acetogenic bacterium Acetobacterium woodii: a coenzyme A loop saves energy for caffeate activation
- PMID: 23315745
- PMCID: PMC3592220
- DOI: 10.1128/AEM.03604-12
Caffeate respiration in the acetogenic bacterium Acetobacterium woodii: a coenzyme A loop saves energy for caffeate activation
Abstract
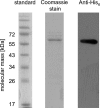
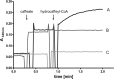
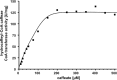
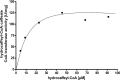
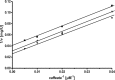
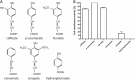
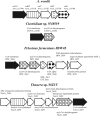
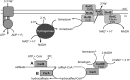
The anaerobic acetogenic bacterium Acetobacterium woodii couples reduction of caffeate with electrons derived from molecular hydrogen to the synthesis of ATP by a chemiosmotic mechanism with sodium ions as coupling ions. Caffeate is activated to caffeyl coenzyme A (caffeyl-CoA) prior to its reduction, and the caffeate reduction operon encodes an ATP-dependent caffeyl-CoA synthetase that is thought to catalyze the initial caffeate activation. The operon also encodes a potential CoA transferase, the product of carA, which was thought to be involved in subsequent ATP-independent caffeate activation. To prove the proposed function of carA, we overproduced its protein in Escherichia coli and then purified it. Purified CarA drives the formation of caffeyl-CoA from caffeate with hydrocaffeyl-CoA as the CoA donor. The dependence of the reaction on caffeate and hydrocaffeyl-CoA followed Michaelis-Menten kinetics, with apparent K(m) values of 75 ± 5 μM for caffeate and 8 ± 2 μM for hydrocaffeyl-CoA. The enzyme activity had broad ranges of pH and temperature optima. In addition to being able to use caffeate, CarA could use p-coumarate and ferulate but not cinnamate, sinapate, or p-hydroxybenzoate as a CoA acceptor. Neither acetyl-CoA nor butyryl-CoA served as the CoA donor for CarA. The enzyme uses a ping-pong mechanism for CoA transfer and is the first classified member of a new subclass of family I CoA transferases that has two catalytic domains on one polypeptide chain. Apparently, CarA catalyzes an energy-saving CoA loop for caffeate activation in the steady state of caffeate respiration.
Figures
Similar articles
-
A caffeyl-coenzyme A synthetase initiates caffeate activation prior to caffeate reduction in the acetogenic bacterium Acetobacterium woodii.J Bacteriol. 2011 Feb;193(4):971-8. doi: 10.1128/JB.01126-10. Epub 2010 Dec 3. J Bacteriol. 2011. PMID: 21131487 Free PMC article.
-
Dissection of the caffeate respiratory chain in the acetogen Acetobacterium woodii: identification of an Rnf-type NADH dehydrogenase as a potential coupling site.J Bacteriol. 2007 Nov;189(22):8145-53. doi: 10.1128/JB.01017-07. Epub 2007 Sep 14. J Bacteriol. 2007. PMID: 17873051 Free PMC article.
-
Dual feedback inhibition of ATP-dependent caffeate activation economizes ATP in caffeate-dependent electron bifurcation.Appl Environ Microbiol. 2024 Sep 18;90(9):e0060224. doi: 10.1128/aem.00602-24. Epub 2024 Aug 23. Appl Environ Microbiol. 2024. PMID: 39177329 Free PMC article.
-
The ins and outs of Na(+) bioenergetics in Acetobacterium woodii.Biochim Biophys Acta. 2009 Jun;1787(6):691-6. doi: 10.1016/j.bbabio.2008.12.015. Epub 2009 Jan 8. Biochim Biophys Acta. 2009. PMID: 19167341 Review.
-
New Horizons in Acetogenic Conversion of One-Carbon Substrates and Biological Hydrogen Storage.Trends Biotechnol. 2019 Dec;37(12):1344-1354. doi: 10.1016/j.tibtech.2019.05.008. Epub 2019 Jun 27. Trends Biotechnol. 2019. PMID: 31257058 Review.
Cited by
-
CO Metabolism in the Acetogen Acetobacterium woodii.Appl Environ Microbiol. 2015 Sep 1;81(17):5949-56. doi: 10.1128/AEM.01772-15. Epub 2015 Jun 19. Appl Environ Microbiol. 2015. PMID: 26092462 Free PMC article.
-
Microbial polyphenol metabolism is part of the thawing permafrost carbon cycle.Nat Microbiol. 2024 Jun;9(6):1454-1466. doi: 10.1038/s41564-024-01691-0. Epub 2024 May 28. Nat Microbiol. 2024. PMID: 38806673 Free PMC article.
-
An electron-bifurcating caffeyl-CoA reductase.J Biol Chem. 2013 Apr 19;288(16):11304-11. doi: 10.1074/jbc.M112.444919. Epub 2013 Mar 11. J Biol Chem. 2013. PMID: 23479729 Free PMC article.
-
A new metabolic trait in an acetogen: Mixed acid fermentation of fructose in a methylene-tetrahydrofolate reductase mutant of Acetobacterium woodii.Environ Microbiol Rep. 2023 Oct;15(5):339-351. doi: 10.1111/1758-2229.13160. Epub 2023 May 7. Environ Microbiol Rep. 2023. PMID: 37150590 Free PMC article.
-
It does not always take two to tango: "Syntrophy" via hydrogen cycling in one bacterial cell.ISME J. 2020 Jun;14(6):1561-1570. doi: 10.1038/s41396-020-0627-1. Epub 2020 Mar 16. ISME J. 2020. PMID: 32203116 Free PMC article.
References
-
- Müller V, Imkamp F, Rauwolf A, Küsel K, Drake HL. 2004. Molecular and cellular biology of acetogenic bacteria, p 251–281 In Nakano MM, Zuber P. (ed), Strict and facultative anaerobes. Medical and environmental aspects. Horizon Biosciences, Norfolk, United Kingdom
-
- Ljungdahl LG. 1994. The acetyl-CoA pathway and the chemiosmotic generation of ATP during acetogenesis, p 63–87 In Drake HL. (ed), Acetogenesis. Chapman & Hall, New York, NY
-
- Ljungdahl LG. 1986. The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu. Rev. Microbiol. 40:415–450 - PubMed
-
- Ragsdale SW. 1997. The eastern and western branches of the Wood/Ljungdahl pathway: how the east and west were won. Biofactors 6:3–11 - PubMed
-
- Ragsdale SW. 1991. Enzymology of the acetyl-CoA pathway of autotrophic CO2 fixation. Crit. Rev. Biochem. Mol. Biol. 26:261–300 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

