Tracking quantum dot-tagged calcium channels at vertebrate photoreceptor synapses: retinal slices and dissociated cells
- PMID: 23315944
- PMCID: PMC3707139
- DOI: 10.1002/0471142301.ns0218s62
Tracking quantum dot-tagged calcium channels at vertebrate photoreceptor synapses: retinal slices and dissociated cells
Abstract
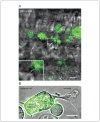
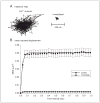
At synapses in the central nervous system, precisely localized assemblies of presynaptic proteins, neurotransmitter-filled vesicles, and postsynaptic receptors are required to communicate messages between neurons. Our understanding of synaptic function has been significantly advanced using electrophysiological methods, but the dynamic spatial behavior and real-time organization of synapses remains poorly understood. In this unit, we describe a method for labeling individual presynaptic calcium channels with photostable quantum dots for single-particle tracking analysis. We have used this technique to examine the mobility of L-type calcium channels in the presynaptic membrane of rod and cone photoreceptors in the retina. These channels control release of glutamate-filled synaptic vesicles at the ribbon synapses in photoreceptor terminals. This technique offers the advantage of providing a real-time biophysical readout of ion channel mobility and can be manipulated by pharmacological or electrophysiological methods. For example, the combination of electrophysiological and single-particle tracking experiments has revealed that fusion of nearby vesicles influences calcium channel mobility and changes in channel mobility can influence release. These approaches can also be readily adapted to examine membrane proteins in other systems.
Figures
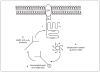
References
-
- Alcor D, Gouzer G, Triller A. Single-particle tracking methods for the study of membrane receptors dynamics. Eur J Neurosci. 2009;30:987–997. - PubMed
-
- Bannai H, Levi S, Schweizer C, Dahan M, Triller A. Imaging the lateral diffusion of membrane molecules with quantum dots. Nat Protoc. 2006;1:2628–2634. - PubMed
-
- Bannai H, Levi S, Schweizer C, Inoue T, Launey T, Racine V, Sibarita JB, Mikoshiba K, Triller A. Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics. Neuron. 2009;62:670–682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

