An anti-angiogenic reverse thermal gel as a drug-delivery system for age-related wet macular degeneration
- PMID: 23316011
- PMCID: PMC4142681
- DOI: 10.1002/mabi.201200384
An anti-angiogenic reverse thermal gel as a drug-delivery system for age-related wet macular degeneration
Abstract
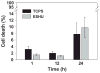
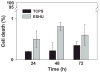
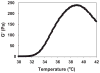
Reverse thermal gels have numerous biomedical implications, as they undergo physical gelation upon temperature increases and can incorporate biomolecules to promote tissue repair. Such a material is developed for the sustained release of bevacizumab (Avastin), a drug used to treat age-related macular degeneration. The polymer, poly(ethylene glycol)-poly(serinol hexamethylene urethane) (ESHU), forms a physical gel when heated to 37 °C and shows good cytocompatibility with ocular cells. ESHU is capable of sustaining bevacizumab release over 17 weeks in vitro, and the release kinetics can be altered by changing the drug dose and the ESHU concentration. These results suggest that ESHU is biologically safe, and suitable for ocular drug delivery.
Copyright © 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



References
-
- Couch SM, Bakri SJ. Semin Ophthalmol. 2011;26:114. - PubMed
-
- El-Mollayess GM, Noureddine BN, Bashshur ZF. Semin Ophthalmol. 2011;26:69. - PubMed
-
- Prasad PS, Schwartz SD, Hubschman JP. Maturitas. 66:46. - PubMed
-
- Anderson OA, Bainbridge JWB, Shima DT. Drug Discovery Today. 2010;15:272. - PubMed
-
- Do DV. Ophthalmology. 2009;116:S24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

