Adaptive evolution of the Streptococcus pyogenes regulatory aldolase LacD.1
- PMID: 23316044
- PMCID: PMC3592009
- DOI: 10.1128/JB.01997-12
Adaptive evolution of the Streptococcus pyogenes regulatory aldolase LacD.1
Abstract
In the human-pathogenic bacterium Streptococcus pyogenes, the tagatose bisphosphate aldolase LacD.1 likely originated through a gene duplication event and was adapted to a role as a metabolic sensor for regulation of virulence gene transcription. Although LacD.1 retains enzymatic activity, its ancestral metabolic function resides in the LacD.2 aldolase, which is required for the catabolism of galactose. In this study, we compared these paralogous proteins to identify characteristics correlated with divergence and novel function. Surprisingly, despite the fact that these proteins have identical active sites and 82% similarity in amino acid sequence, LacD.1 was less efficient at cleaving both fructose and tagatose bisphosphates. Analysis of kinetic properties revealed that LacD.1's adaptation was associated with a decrease in k(cat) and an increase in K(m). Construction and analysis of enzyme chimeras indicated that non-active-site residues previously associated with the variable activities of human aldolase isoenzymes modulated LacD.1's affinity for substrate. Mutant LacD.1 proteins engineered to have LacD.2-like levels of enzymatic efficiency lost the ability to function as regulators, suggesting that an alteration in efficiency was required for adaptation. In competition under growth conditions that mimic a deep-tissue environment, LacD.1 conferred a significant gain in fitness that was associated with its regulatory activity. Taken together, these data suggest that LacD.1's adaptation represents a form of neofunctionalization in which duplication facilitated the gain of regulatory function important for growth in tissue and pathogenesis.
Figures


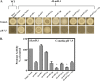
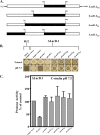

References
-
- Kim JW, Dang CV. 2005. Multifaceted roles of glycolytic enzymes. Trends Biochem. Sci. 30:142–150 - PubMed
-
- Lohr D, Venkov P, Zlatanova J. 1995. Transcriptional regulation in the yeast GAL gene family: a complex genetic network. FASEB J. 9:777–787 - PubMed
-
- Hahn MW. 2009. Distinguishing among evolutionary models for the maintenance of gene duplicates. J. Hered. 100:605–617 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

