Deletion of vacuolar proton-translocating ATPase V(o)a isoforms clarifies the role of vacuolar pH as a determinant of virulence-associated traits in Candida albicans
- PMID: 23316054
- PMCID: PMC3585055
- DOI: 10.1074/jbc.M112.426197
Deletion of vacuolar proton-translocating ATPase V(o)a isoforms clarifies the role of vacuolar pH as a determinant of virulence-associated traits in Candida albicans
Abstract
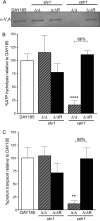
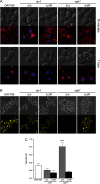
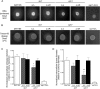
Vacuolar proton-translocating ATPase (V-ATPase) is a central regulator of cellular pH homeostasis, and inactivation of all V-ATPase function has been shown to prevent infectivity in Candida albicans. V-ATPase subunit a of the Vo domain (Voa) is present as two fungal isoforms: Stv1p (Golgi) and Vph1p (vacuole). To delineate the individual contribution of Stv1p and Vph1p to C. albicans physiology, we created stv1Δ/Δ and vph1Δ/Δ mutants and compared them to the corresponding reintegrant strains (stv1Δ/ΔR and vph1Δ/ΔR). V-ATPase activity, vacuolar physiology, and in vitro virulence-related phenotypes were unaffected in the stv1Δ/Δ mutant. The vph1Δ/Δ mutant exhibited defective V1Vo assembly and a 90% reduction in concanamycin A-sensitive ATPase activity and proton transport in purified vacuolar membranes, suggesting that the Vph1p isoform is essential for vacuolar V-ATPase activity in C. albicans. The vph1Δ/Δ cells also had abnormal endocytosis and vacuolar morphology and an alkalinized vacuolar lumen (pHvph1Δ/Δ = 6.8 versus pHvph1Δ/ΔR = 5.8) in both yeast cells and hyphae. Secreted protease and lipase activities were significantly reduced, and M199-induced filamentation was impaired in the vph1Δ/Δ mutant. However, the vph1Δ/Δ cells remained competent for filamentation induced by Spider media and YPD, 10% FCS, and biofilm formation and macrophage killing were unaffected in vitro. These studies suggest that different virulence mechanisms differentially rely on acidified vacuoles and that the loss of both vacuolar (Vph1p) and non-vacuolar (Stv1p) V-ATPase activity is necessary to affect in vitro virulence-related phenotypes. As a determinant of C. albicans pathogenesis, vacuolar pH alone may prove less critical than originally assumed.
Figures


References
-
- Manolson M. F., Proteau D., Jones E. W. (1992) Evidence for a conserved 95–120-kDa subunit associated with and essential for activity of V-ATPases. J. Exp. Biol. 172, 105–112 - PubMed
-
- Manolson M. F., Proteau D., Preston R. A., Stenbit A., Roberts B. T., Hoyt M. A., Preuss D., Mulholland J., Botstein D., Jones E. W. (1992) The VPH1 gene encodes a 95-kDa integral membrane polypeptide required for in vivo assembly and activity of the yeast vacuolar H+-ATPase. J. Biol. Chem. 267, 14294–14303 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

