Increased expression of senescence markers in cystic fibrosis airways
- PMID: 23316069
- PMCID: PMC3602742
- DOI: 10.1152/ajplung.00091.2012
Increased expression of senescence markers in cystic fibrosis airways
Abstract
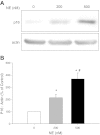
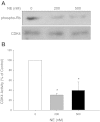
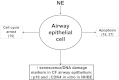
Cystic Fibrosis (CF) is a chronic lung disease characterized by chronic neutrophilic airway inflammation and increased levels of neutrophil elastase (NE) in the airways. We have previously reported that NE treatment triggers cell cycle arrest. Cell cycle arrest can lead to senescence, a complete loss of replicative capacity. Importantly, senescent cells can be proinflammatory and would perpetuate CF chronic inflammation. By immunohistochemistry, we evaluated whether airway sections from CF and control subjects expressed markers of senescence, including p16(INK4a) (p16), a cyclin-dependent kinase inhibitor, phospho-Histone H2A.X (γH2A.X), and phospho-checkpoint 2 kinase (phospho-Chk2), which are also DNA damage response markers. Compared with airway epithelium from control subjects, CF airway epithelium had increased levels of expression of all three senescence markers. We hypothesized that the high load of NE in the CF airway triggers epithelial senescence by upregulating expression of p16, which inhibits cyclin-dependent kinase 4 (CDK4). Normal human bronchial epithelial (NHBE) cells, cultured in air-liquid interface were treated with NE (0, 200, and 500 nM) to induce visible injury. Total cell lysates were collected and evaluated by Western analysis for p16 protein expression and CDK4 kinase activity. NE significantly increased p16 expression and decreased CDK4 kinase activity in NHBE cells. These results support the concept that NE triggers expression of senescence markers in CF airway epithelial cells.
Figures



References
-
- Aoshiba K, Yasuda K, Yahui S, Tamaoki J, Nagai A. Serine proteases increase oxidative stress in lung cells. Am J Physiol Lung Cell Mol Physiol 281: L556–L564, 2001 - PubMed
-
- Barash H, ERG , Edrei Y, Ella E, Israel A, Cohen I, Corchia N, Ben-Moshe T, Pappo O, Pikarsky E, Goldenberg D, Shiloh Y, Galun E, Abramovitch R. Accelerated carcinogenesis following liver regeneration is associated with chronic inflammation-induced double-strand DNA breaks. Proc Natl Acad Sci USA 107: 2207–2212, 2010 - PMC - PubMed
-
- Birrer P, McElvaney NG, Rudeberg A, Sommer CW, Liechti-Gallati S, Kraemer R, Hubbard R, Crystal RG. Protease-antiprotease imbalance in the lungs of children with cystic fibrosis. Am J Respir Crit Care Med 150: 207–213, 1994 - PubMed
-
- Brennan S, Sly PD, Gangell CL, Sturges N, Winfield K, Wikstrom M, Gard S, Upham JW. Alveolar macrophages and CC chemokines are increased in children with cystic fibrosis. Eur Respir J 34: 655–661, 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

