Adalimumab, a human anti-TNF monoclonal antibody, outcome study for the prevention of joint damage in Japanese patients with early rheumatoid arthritis: the HOPEFUL 1 study
- PMID: 23316080
- PMCID: PMC4151516
- DOI: 10.1136/annrheumdis-2012-202433
Adalimumab, a human anti-TNF monoclonal antibody, outcome study for the prevention of joint damage in Japanese patients with early rheumatoid arthritis: the HOPEFUL 1 study
Abstract
Objectives: To evaluate the efficacy and safety of adalimumab+methotrexate (MTX) in Japanese patients with early rheumatoid arthritis (RA) who had not previously received MTX or biologics.
Methods: This randomised, double-blind, placebo-controlled, multicentre study evaluated adalimumab 40 mg every other week+MTX 6-8 mg every week versus MTX 6-8 mg every week alone for 26 weeks in patients with RA (≤2-year duration). The primary endpoint was inhibition of radiographic progression (change (Δ) from baseline in modified total Sharp score (mTSS)) at week 26.
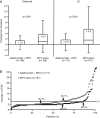
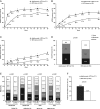
Results: A total of 171 patients received adalimumab+MTX (mean dose, 6.2±0.8 mg/week) and 163 patients received MTX alone (mean dose, 6.6±0.6 mg/week, p<0.001). The mean RA duration was 0.3 years and 315 (94.3%) had high disease activity (DAS28>5.1). Adalimumab+MTX significantly inhibited radiographic progression at week 26 versus MTX alone (ΔmTSS, 1.5±6.1 vs 2.4±3.2, respectively; p<0.001). Significantly more patients in the adalimumab+MTX group (62.0%) did not show radiographic progression (ΔmTSS≤0.5) versus the MTX alone group (35.4%; p<0.001). Patients treated with adalimumab+MTX were significantly more likely to achieve American College of Rheumatology responses and achieve clinical remission, using various definitions, at 26 weeks versus MTX alone. Combination therapy was well tolerated, and no new safety signals were observed.
Conclusions: Adalimumab in combination with low-dose MTX was well tolerated and efficacious in suppressing radiographic progression and improving clinical outcomes in Japanese patients with early RA and high disease activity.
Keywords: Anti-TNF; Methotrexate; Rheumatoid Arthritis.
Figures
References
-
- Filipovic I, Walker D, Forster F, et al. Quantifying the economic burden of productivity loss in rheumatoid arthritis. Rheumatology (Oxford) 2011; 50:1083–90 - PubMed
-
- Scott DL, Wolfe F, Huizinga TWJ. Rheumatoid arthritis. Lancet 2010;376:1094–108 - PubMed
-
- Takeuchi T. Revolutionary change in rheumatoid arthritis management with biological therapy. Keio J Med 2011;60:75–81 - PubMed
-
- Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 2008;59:762–84 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

