Osteoconductivity and Hydrophilicity of TiO(2) Coatings on Ti Substrates Prepared by Different Oxidizing Processes
- PMID: 23316128
- PMCID: PMC3535825
- DOI: 10.1155/2012/495218
Osteoconductivity and Hydrophilicity of TiO(2) Coatings on Ti Substrates Prepared by Different Oxidizing Processes
Abstract
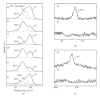
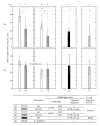
Various techniques for forming TiO(2) coatings on Ti have been investigated for the improvement of the osteoconductivity of Ti implants. However, it is not clear how the oxidizing process affects this osteoconductivity. In this study, TiO(2) coatings were prepared using the following three processes: anodizing in 0.1 M H(3)PO(4) or 0.1 M NaOH aqueous solution; thermal oxidation at 673 K for 2 h in air; and a two-step process of anodizing followed by thermal oxidation. The oxide coatings were evaluated using SEM, XRD, and XPS. The water contact angle on the TiO(2) coatings was measured as a surface property. The osteoconductivity of these samples was evaluated by measuring the contact ratio of formed hard tissue on the implanted samples (defined as the R(B-I) value) after 14 d implantation in rats' tibias. Anatase was formed by anodizing and rutile by thermal oxidation, but the difference in the TiO(2) crystal structure did not influence the osteoconductivity. Anodized TiO(2) coatings were hydrophilic, but thermally oxidized TiO(2) coatings were less hydrophilic than anodized TiO(2) coatings because they lacked in surface OH groups. The TiO(2) coating process using anodizing without thermal oxidation gave effective improvement of the osteoconductivity of Ti samples.
Figures



Similar articles
-
Improvement of Corrosion Resistance of TiO2 Layers in Strong Acidic Solutions by Anodizing and Thermal Oxidation Treatment.Materials (Basel). 2021 Mar 3;14(5):1188. doi: 10.3390/ma14051188. Materials (Basel). 2021. PMID: 33802436 Free PMC article.
-
Preparation of TiO(2) layers on cp-Ti and Ti6Al4V by thermal and anodic oxidation and by sol-gel coating techniques and their characterization.J Biomed Mater Res. 2002 Jan;59(1):18-28. doi: 10.1002/jbm.1212. J Biomed Mater Res. 2002. PMID: 11745533
-
Tailoring Surface Hydrophilicity Property for Biomedical 316L and 304 Stainless Steels: A Special Perspective on Studying Osteoconductivity and Biocompatibility.ACS Appl Mater Interfaces. 2019 Dec 11;11(49):45489-45497. doi: 10.1021/acsami.9b17312. Epub 2019 Nov 22. ACS Appl Mater Interfaces. 2019. PMID: 31714730
-
Anodic Voltage Dependence of Ti-6Al-4V Substrates and Hydroxyapatite Coating.J Nanosci Nanotechnol. 2019 Sep 1;19(9):5700-5706. doi: 10.1166/jnn.2019.16530. J Nanosci Nanotechnol. 2019. PMID: 30961727
-
Microstructure and Electrochemical Behavior of TiO₂ Nanotubes Coated on Titanium-Based Substrate Before and After Thermal Treatment.J Nanosci Nanotechnol. 2019 May 1;19(5):2989-2996. doi: 10.1166/jnn.2019.15859. J Nanosci Nanotechnol. 2019. PMID: 30501810
Cited by
-
Non-mulberry silk fibroin influence osteogenesis and osteoblast-macrophage cross talk on titanium based surface.Sci Rep. 2014 Apr 22;4:4745. doi: 10.1038/srep04745. Sci Rep. 2014. PMID: 24752225 Free PMC article.
-
Enhancing Titanium Osteoconductivity by Alkali-Hot Water Treatment.ACS Omega. 2024 Oct 22;9(44):44568-44576. doi: 10.1021/acsomega.4c06702. eCollection 2024 Nov 5. ACS Omega. 2024. PMID: 39524660 Free PMC article.
References
-
- Adell R, Eriksson B, Lekholm U, Brånemark PI, Jemt T. Long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. The International Journal of Oral and Maxillofacial Implants. 1990;5(4):347–359. - PubMed
-
- van Steenberghe D, Lekholm U, Bolender C, et al. Applicability of osseointegrated oral implants in the rehabilitation of partial edentulism: a prospective multicenter study on 558 fixtures. The International Journal of Oral and Maxillofacial Implants. 1990;5(3):272–281. - PubMed
-
- Hench LL, Wilson J. An Introduction to Bioceramics. chapter 1. Singapore: World Scientific; 1993.
-
- Kuroda K, Ichino R, Okido M, Takai O. Effects of ion concentration and pH on hydroxyapatite deposition from aqueous solution onto titanium by the thermal substrate method. Journal of Biomedical Materials Research. 2002;61(3):354–359. - PubMed
-
- Kuroda K, Ichino R, Okido M, Takai O. Effects of ion concentration and pH on hydroxyapatite deposition from aqueous solution onto titanium by the thermal substrate method. Journal of Biomedical Materials Research. 2002;61(3):354–359. - PubMed
LinkOut - more resources
Full Text Sources

