Cortical modulation of auditory processing in the midbrain
- PMID: 23316140
- PMCID: PMC3539853
- DOI: 10.3389/fncir.2012.00114
Cortical modulation of auditory processing in the midbrain
Abstract
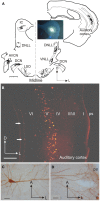
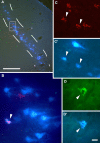
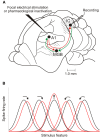
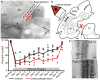
In addition to their ascending pathways that originate at the receptor cells, all sensory systems are characterized by extensive descending projections. Although the size of these connections often outweighs those that carry information in the ascending auditory pathway, we still have a relatively poor understanding of the role they play in sensory processing. In the auditory system one of the main corticofugal projections links layer V pyramidal neurons with the inferior colliculus (IC) in the midbrain. All auditory cortical fields contribute to this projection, with the primary areas providing the largest outputs to the IC. In addition to medium and large pyramidal cells in layer V, a variety of cell types in layer VI make a small contribution to the ipsilateral corticocollicular projection. Cortical neurons innervate the three IC subdivisions bilaterally, although the contralateral projection is relatively small. The dorsal and lateral cortices of the IC are the principal targets of corticocollicular axons, but input to the central nucleus has also been described in some studies and is distinctive in its laminar topographic organization. Focal electrical stimulation and inactivation studies have shown that the auditory cortex can modify almost every aspect of the response properties of IC neurons, including their sensitivity to sound frequency, intensity, and location. Along with other descending pathways in the auditory system, the corticocollicular projection appears to continually modulate the processing of acoustical signals at subcortical levels. In particular, there is growing evidence that these circuits play a critical role in the plasticity of neural processing that underlies the effects of learning and experience on auditory perception by enabling changes in cortical response properties to spread to subcortical nuclei.
Keywords: auditory cortex; corticofugal; descending projection; inferior colliculus; plasticity; sound localization.
Figures





References
-
- Aitkin L. M., Phillips S. C. (1984). Is the inferior colliculus an obligatory relay in the cat auditory system? Neurosci. Lett. 44, 259–264 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

