Automatic quantification of in vitro NET formation
- PMID: 23316198
- PMCID: PMC3540390
- DOI: 10.3389/fimmu.2012.00413
Automatic quantification of in vitro NET formation
Abstract
Neutrophil Extracellular Traps (NETs) consist of decondensed chromatin studded with granular and some cytoplasmic proteins. They are formed by activated neutrophil granulocytes, also called polymorphonuclear leukocytes (PMN) as the result of an active cell death program, named NETosis. NETosis can be induced by a wide range of stimuli including coculture of neutrophils with pathogens (bacteria, fungi, parasites, virus particles), activated platelets, or pathogen components. The first step of the NETotic cascade is stimulation of one or several receptors followed by activation of the Raf/MEK/ERK pathway that culminates in the assembly of the multimeric NADPH oxidase complex and the production of reactive oxygen species (ROS). Later, intracellular membranes disintegrate, the granular protein Neutrophil Elastase enters the nucleus and processes core histones that also get hypercitrullinated. This leads to decondensation and mobilization of chromatin. The amount of NET formation varies with the degree of stimulation, and this is dependent on the type and concentration of the stimulus. NETs can be quantified using various methods including fluorescence microscopy or measuring DNA release. Each of these methods have specific drawbacks: analysis of fluorescence microscopy is prone to subjective variations, and DNA release does not differentiate between DNA that has been released by NETosis or by other forms of cell death. Here we present a protocol to semi-automatically quantify NET formation. It relies on the observation that anti-chromatin antibodies bind more readily to decondensed chromatin present in the nuclei of cells undergoing NETosis and in the NETs. Relating the fluorescence signals of the anti-chromatin antibody to the signals of a DNA-binding dye allows the automatic calculation of the percentage of netting neutrophils. This method does not require sophisticated microscopic equipment, and the images are quantified with a public-domain software package.
Keywords: NEtosis; chromatin; immunofluorescence; quantification; segmentation.
Figures




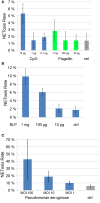
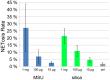
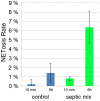
References
-
- Aga E., Katschinski D. M., van Zandbergen G., Laufs H., Hansen B., Muller K., et al. (2002). Inhibition of the spontaneous apoptosis of neutrophil granulocytes by the intracellular parasite Leishmania major. J. Immunol. 169, 898–905 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

