Sphingolipids: a potential molecular approach to treat allergic inflammation
- PMID: 23316248
- PMCID: PMC3536436
- DOI: 10.1155/2012/154174
Sphingolipids: a potential molecular approach to treat allergic inflammation
Abstract
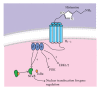
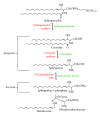
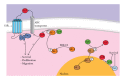
Allergic inflammation is an immune response to foreign antigens, which begins within minutes of exposure to the allergen followed by a late phase leading to chronic inflammation. Prolonged allergic inflammation manifests in diseases such as urticaria and rhino-conjunctivitis, as well as chronic asthma and life-threatening anaphylaxis. The prevalence of allergic diseases is profound with 25% of the worldwide population affected and a rising trend across all ages, gender, and racial groups. The identification and avoidance of allergens can manage this disease, but this is not always possible with triggers being common foods, prevalent air-borne particles and only extremely low levels of allergen exposure required for sensitization. Patients who are sensitive to multiple allergens require prophylactic and symptomatic treatments. Current treatments are often suboptimal and associated with adverse effects, such as the interruption of cognition, sleep cycles, and endocrine homeostasis, all of which affect quality of life and are a financial burden to society. Clearly, a better therapeutic approach for allergic diseases is required. Herein, we review the current knowledge of allergic inflammation and discuss the role of sphingolipids as potential targets to regulate inflammatory development in vivo and in humans. We also discuss the benefits and risks of using sphingolipid inhibitors.
Figures

References
-
- Jiang P, Liu J, Yan XB, Liu RY. Several interleukin-4 and interleukin-13 gene single nucleotide polymorphisms among Chinese asthmatic patients. Allergy and Asthma Proceedings. 2009;30(4):413–418. - PubMed
-
- Kay AB. Allergy and allergic diseases. First of two parts. New England Journal of Medicine. 2001;344(1):30–37. - PubMed
-
- Hakim-Rad K, Metz M, Maurer M. Mast cells: makers and breakers of allergic inflammation. Current Opinion in Allergy and Clinical Immunology. 2009;9(5):427–430. - PubMed
-
- Grimbaldeston MA, Metz M, Yu M, Tsai M, Galli SJ. Effector and potential immunoregulatory roles of mast cells in IgE-associated acquired immune responses. Current Opinion in Immunology. 2006;18(6):751–760. - PubMed
-
- Iriyoshi N, Takeuchi K, Yuta A, Ukai K, Sakakura Y. Increased expression of histamine H1 receptor mRNA in allergic rhinitis. Clinical and Experimental Allergy. 1996;26(4):379–385. - PubMed
LinkOut - more resources
Full Text Sources

