In vitro chondrogenic differentiation of human adipose-derived stem cells with silk scaffolds
- PMID: 23316274
- PMCID: PMC3540700
- DOI: 10.1177/2041731412466405
In vitro chondrogenic differentiation of human adipose-derived stem cells with silk scaffolds
Abstract
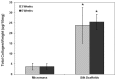
Human adipose-derived stem cells have shown chondrogenic differentiation potential in cartilage tissue engineering in combination with natural and synthetic biomaterials. In the present study, we hypothesized that porous aqueous-derived silk protein scaffolds would be suitable for chondrogenic differentiation of human adipose-derived stem cells. Human adipose-derived stem cells were cultured up to 6 weeks, and cell proliferation and chondrogenic differentiation were investigated and compared with those in conventional micromass culture. Cell proliferation, glycosaminoglycan, and collagen levels in aqueous-derived silk scaffolds were significantly higher than in micromass culture. Transcript levels of SOX9 and type II collagen were also upregulated in the cell-silk constructs at 6 weeks. Histological examination revealed that the pores of the silk scaffolds were filled with cells uniformly distributed. In addition, chondrocyte-specific lacunae formation was evident and distributed in the both groups. The results suggest the biodegradable and biocompatible three-dimensional aqueous-derived silk scaffolds provided an improved environment for chondrogenic differentiation compared to micromass culture.
Keywords: adipose-derived stem cells; cartilage; chondrogenic differentiation; silk scaffolds.
Figures




References
-
- Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage 2002; 10(6): 432–463 - PubMed
-
- Ficat RP, Ficat C, Gedeon P, et al. Spongialization: new treatment for diseased patellae. Clin Orthop Relat Res 1979; 144: 74–83 - PubMed
-
- Mitchell N, Shepard N. Resurfacing of adult rabbit articular cartilage by multiple perforations through subchondral bone. J Bone Joint Surg Am 1976; 58(2): 230–233 - PubMed
-
- Levy AS, Lohnes J, Sculley S, et al. Chondral delamination of the knee in soccer players. Am J Sports Med 1996; 24(5): 634–639 - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

