Protein kinase g iα inhibits pressure overload-induced cardiac remodeling and is required for the cardioprotective effect of sildenafil in vivo
- PMID: 23316302
- PMCID: PMC3541610
- DOI: 10.1161/JAHA.112.003731
Protein kinase g iα inhibits pressure overload-induced cardiac remodeling and is required for the cardioprotective effect of sildenafil in vivo
Abstract
Background: Cyclic GMP (cGMP) signaling attenuates cardiac remodeling, but it is unclear which cGMP effectors mediate these effects and thus might serve as novel therapeutic targets. Therefore, we tested whether the cGMP downstream effector, cGMP-dependent protein kinase G Iα (PKGIα), attenuates pressure overload-induced remodeling in vivo.
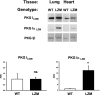
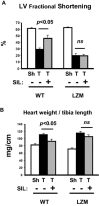
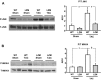
Methods and results: The effect of transaortic constriction (TAC)-induced left ventricular (LV) pressure overload was examined in mice with selective mutations in the PKGIα leucine zipper interaction domain. Compared with wild-type littermate controls, in response to TAC, these Leucine Zipper Mutant (LZM) mice developed significant LV systolic and diastolic dysfunction by 48 hours (n=6 WT sham, 6 WT TAC, 5 LZM sham, 9 LZM TAC). In response to 7-day TAC, the LZM mice developed increased pathologic hypertrophy compared with controls (n=5 WT sham, 4 LZM sham, 8 WT TAC, 11 LZM TAC). In WT mice, but not in LZM mice, phosphodiesterase 5 (PDE5) inhibition with sildenafil (Sil) significantly inhibited TAC-induced cardiac hypertrophy and LV systolic dysfunction in WT mice, but this was abolished in the LZM mice (n=3 WT sham, 4 LZM sham, 3 WT TAC vehicle, 6 LZM TAC vehicle, 4 WT TAC Sil, 6 LZM TAC Sil). And in response to prolonged, 21-day TAC (n=8 WT sham, 7 LZM sham, 21 WT TAC, 15 LZM TAC), the LZM mice developed markedly accelerated mortality and congestive heart failure. TAC induced activation of JNK, which inhibits cardiac remodeling in vivo, in WT, but not in LZM, hearts, identifying a novel signaling pathway activated by PKGIα in the heart in response to LV pressure overload.
Conclusions: These findings reveal direct roles for PKGIα in attenuating pressure overload-induced remodeling in vivo and as a required effector for the cardioprotective effects of sildenafil.
Keywords: heart failure; nitric oxide; protein kinase G; remodeling heart failure; signal transduction.
Figures



References
-
- Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:215-222. - PubMed
-
- Scherrer-Crosbie M, Ullrich K, Bloch KD, Nakajima H, Nasseri B, Aretz HT, Lindsey ML, Vançon A-C, Huang PL, Lee RT, Zapol WM, Picard MH. Endothelial nitric oxide synthase limits left ventricular remodeling after myocardial infarction in mice. Circulation. 2001;104:1286-1291. - PubMed
-
- Ichinose F, Bloch KD, Wu JC, Hataishi R, Aretz HT, Picard MH, Scherrer-Crosbie M. Pressure overload-induced LV hypertrophy and dysfunction in mice are exacerbated by congenital NOS3 deficiency. Am J Physiol Heart Circ Physiol. 2004;286:H1070-H1075. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

