Deleterious effect of the IL-23/IL-17A axis and γδT cells on left ventricular remodeling after myocardial infarction
- PMID: 23316306
- PMCID: PMC3541626
- DOI: 10.1161/JAHA.112.004408
Deleterious effect of the IL-23/IL-17A axis and γδT cells on left ventricular remodeling after myocardial infarction
Abstract
Background: Left ventricular (LV) remodeling leads to chronic heart failure and is a main determinant of morbidity and mortality after myocardial infarction (MI). At the present time, therapeutic options to prevent LV remodeling are limited.
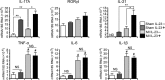
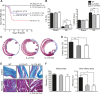
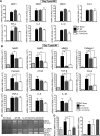
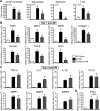
Methods and results: We created a large MI by permanent ligation of the coronary artery and identified a potential link between the interleukin (IL)-23/IL-17A axis and γδT cells that affects late-stage LV remodeling after MI. Despite the finsinf that infarct size 24 hours after surgery was similar to that in wild-type mice, a deficiency in IL-23, IL-17A, or γδT cells improved survival after 7 days, limiting infarct expansion and fibrosis in noninfarcted myocardium and alleviating LV dilatation and systolic dysfunction on day 28 post-MI. M(1) macrophages and neutrophils were the major cellular source of IL-23, whereas >90% of IL-17A-producing T cells in infarcted heart were CD4(-) TCRγδ(+) (γδT) cells. Toll-like receptor signaling and IL-1β worked in concert with IL-23 to drive expansion and IL-17A production in cardiac γδT cells, whereas the sphingosine-1-phosphate receptor and CCL20/CCR6 signaling pathways mediated γδT cell recruitment into infarcted heart. IL-17A was not involved in the acute inflammatory response, but it functioned specifically in the late remodeling stages by promoting sustained infiltration of neutrophils and macrophages, stimulating macrophages to produce proinflammatory cytokines, aggravating cardiomyocyte death, and enhancing fibroblast proliferation and profibrotic gene expression.
Conclusions: The IL-23/IL-17A immune axis and γδT cells are potentially promising therapeutic targets after MI to prevent progression to end-stage dilated cardiomyopathy.
Keywords: heart failure; immune system; inflammation; myocardial infarction; remodeling.
Figures










References
-
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012;125:e2-e220. - PMC - PubMed
-
- Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet. 2006;367:356-367. - PubMed
-
- Krum H, Teerlink JR. Medical therapy for chronic heart failure. Lancet. 2011;378:713-721. - PubMed
-
- Mudd JO, Kass DA. Tackling heart failure in the twenty-first century. Nature. 2008;451:919-928. - PubMed
-
- Brown NJ, Vaughan DE. Angiotensin-converting enzyme inhibitors. Circulation. 1998;97:1411-1420. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

