In vivo cardiac cellular reprogramming efficacy is enhanced by angiogenic preconditioning of the infarcted myocardium with vascular endothelial growth factor
- PMID: 23316332
- PMCID: PMC3540681
- DOI: 10.1161/JAHA.112.005652
In vivo cardiac cellular reprogramming efficacy is enhanced by angiogenic preconditioning of the infarcted myocardium with vascular endothelial growth factor
Abstract
Background: In situ cellular reprogramming offers the possibility of regenerating functional cardiomyocytes directly from scar fibroblasts, obviating the challenges of cell implantation. We hypothesized that pretreating scar with gene transfer of the angiogenic vascular endothelial growth factor (VEGF) would enhance the efficacy of this strategy.
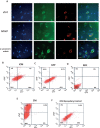
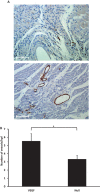
Methods and results: Gata4, Mef2c, and Tbx5 (GMT) administration via lentiviral transduction was demonstrated to transdifferentiate rat fibroblasts into (induced) cardiomyocytes in vitro by cardiomyocyte marker studies. Fisher 344 rats underwent coronary ligation and intramyocardial administration of an adenovirus encoding all 3 major isoforms of VEGF (AdVEGF-All6A(+)) or an AdNull control vector (n=12/group). Lentivirus encoding GMT or a GFP control was administered to each animal 3 weeks later, followed by histologic and echocardiographic analyses. GMT administration reduced the extent of fibrosis by half compared with GFP controls (12 ± 2% vs 24 ± 3%, P<0.01) and reduced the number of myofibroblasts detected in the infarct zone by 4-fold. GMT-treated animals also demonstrated greater density of cardiomyocyte-specific marker beta myosin heavy chain 7(+) cells compared with animals receiving GFP with or without VEGF (P<0.01). Ejection fraction was significantly improved after GMT vs GFP administration (12 ± 3% vs -7 ± 3%, P<0.01). Eight (73%) GFP animals but no GMT animals demonstrated decreased ejection fraction during this interval (P<0.01). Also, improvement in ejection fraction was 4-fold greater in GMT/VEGF vs GMT/null animals (17 ± 2% vs 4 ± 1%, P<0.05).
Conclusions: VEGF administration to infarcted myocardium enhances the efficacy of GMT-mediated cellular reprogramming in improving myocardial function and reducing the extent of myocardial fibrosis compared with the use of GMT or VEGF alone.
Keywords: angiogenesis; gene therapy; myocardial infarction; stem cells.
Figures






References
-
- AHA statistical update heart disease and stroke statistics—2009 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009; 119:480-486 - PubMed
-
- Taylor DO, Edwards LB, Boucek MM, Trulock EP, Keck BM, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: twenty‐first official adult heart transplant report. J Heart Lung Transplant. 2004; 23:796-803 - PubMed
-
- Lietz K, Long JW, Kfoury AG, Slaughter MS, Silver MA, Milano CA, Rogers JG, Naka Y, Mancini D, Miller LW. Outcomes of left ventricular assist device implantation as destination therapy in the post‐REMATCH era: implications for patient selection. Circulation. 2007; 116:497-505 - PubMed
-
- Leor J, Patterson M, Quinones MJ, Kedes LH, Kloner RA. Transplantation of fetal myocardial tissue into the infarcted myocardium of rat: a potential method for repair of infarcted myocardium? Circulation. 1996; 94:II332-II336 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

