Prolonged Therapy with Imatinib Mesylate before Surgery for Advanced Gastrointestinal Stromal Tumor Results of a Phase II Trial
- PMID: 23316352
- PMCID: PMC3534224
- DOI: 10.1155/2012/761576
Prolonged Therapy with Imatinib Mesylate before Surgery for Advanced Gastrointestinal Stromal Tumor Results of a Phase II Trial
Abstract
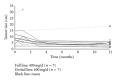
Purpose. Proven efficacy of imatinib mesylate in gastrointestinal stromal tumour (GIST) has led to its use in advanced disease and, more recently, in adjuvant and neoadjuvant settings. The purpose of this study was to evaluate the optimal neoadjuvant imatinib duration to reduce the morbidity of surgery and increase the possibility of resection completeness in advanced tumours. Patients and Method. Patients with advanced GIST were enrolled into a registered open-label multicenter trial and received imatinib daily for a maximum of 12 months, followed by en bloc resection. Data were prospectively collected regarding tumour assessment, response rate, surgical characteristics, recurrence, and survival. Results. Fourteen patients with advanced GIST were enrolled. According to RECIST criteria, 6 patients had partial response and 8 had stable disease. The overall tumour size reduction was 25% (0-62.5%), and there was no tumour progression. Eleven patients underwent tumour resection, and all had R0 resection. After a median followup of 48 months, 4-year OS and DFS were 100% and 64%, respectively. Conclusion. This prospective trial showed that one year of neoadjuvant imatinib in advanced GIST is safe and associated with high rate of complete microscopic resection. It is not associated with increased resistance, progression, or complication rates.
Figures
References
-
- Joensuu H, Fletcher C, Dimitrijevic S, Silberman S, Roberts P, Demetri G. Management of malignant gastrointestinal stromal tumours. Lancet Oncology. 2002;3(11):655–664. - PubMed
-
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577–580. - PubMed
-
- Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299(5607):708–710. - PubMed
-
- Hur K, Lee HJ, Woo JH, Kim JH, Yang HK. Gene expression profiling of human gastrointestinal stromal tumors according to its malignant potential. Digestive Diseases and Sciences. 2010;55(9):2561–2567. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials