Strategies for intranasal delivery of vaccines
- PMID: 23316448
- PMCID: PMC3539070
- DOI: 10.1007/s13346-012-0085-z
Strategies for intranasal delivery of vaccines
Abstract
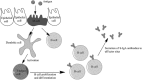
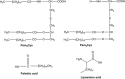
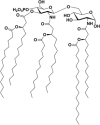
The vast majority of human pathogens colonize and invade at the mucosal surfaces. Preventing infection at these sites via mucosally active vaccines is a promising and rational approach for vaccine development. However, it is only recently that the stimulation of local immunity at the mucosal surfaces has become a primary objective in addition to inducing systemic immunity. This review describes vaccine formulations designed for mucosal delivery to the nasal-associated lymphoid tissue, via intranasal administration. The association of antigens with mucosal adjuvants and delivery systems is emphasised.
Figures


References
-
- Ricciardi W. The old Edward Jenner and the new public health: the future of vaccines in Europe. Eur J Public Health. 2008;18(4):353. - PubMed
-
- Ellner PD. Smallpox: gone but not forgotten. Infection. 1998;26(5):263–269. - PubMed
-
- Almeida AJ, Alpar HO. Nasal delivery of vaccines. J Drug Target. 1996;3(6):455–467. - PubMed
-
- Boyaka PN, Tafaro A, Fischer R, Leppla SH, Fujihashi K, McGhee JR. Effective mucosal immunity to anthrax: neutralizing antibodies and Th cell responses following nasal immunization with protective antigen. J Immunol. 2003;170(11):5636–5643. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

