Strategies for Target-Specific Contrast Agents for Magnetic Resonance Imaging
- PMID: 23316452
- PMCID: PMC3539778
- DOI: 10.2174/2211555211201010012
Strategies for Target-Specific Contrast Agents for Magnetic Resonance Imaging
Abstract
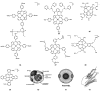
This review describes recent research efforts focused on increasing the specificity of contrast agents for proton magnetic resonance imaging (MRI). Contrast agents play an indispensable role in MRI by enhancing the inherent contrast of images; however, the non-specific nature of current clinical contrast agents limits their usefulness. This limitation can be addressed by conjugating contrast agents or contrast-agent-loaded carriers-including polymers, nanoparticles, dendrimers, and liposomes-to molecules that bind to biological sites of interest. An alternative approach to conjugation is synthetically mimicking biological structures with metal complexes that are also contrast agents. In this review, we describe the advantages and limitations of these two targeting strategies with respect to translation from in vitro to in vivo imaging while focusing on advances from the last ten years.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Pushing the sensitivity envelope of lanthanide-based magnetic resonance imaging (MRI) contrast agents for molecular imaging applications.Acc Chem Res. 2009 Jul 21;42(7):822-31. doi: 10.1021/ar800192p. Acc Chem Res. 2009. PMID: 19534516 Review.
-
Comparing Strategies in the Design of Responsive Contrast Agents for Magnetic Resonance Imaging: A Case Study with Copper and Zinc.Acc Chem Res. 2018 Feb 20;51(2):342-351. doi: 10.1021/acs.accounts.7b00301. Epub 2018 Jan 22. Acc Chem Res. 2018. PMID: 29356506
-
Gadolinium-Loaded Polychelating Polymer-Containing Tumor-Targeted Liposomes.Methods Mol Biol. 2017;1522:179-192. doi: 10.1007/978-1-4939-6591-5_14. Methods Mol Biol. 2017. PMID: 27837539
-
Nanotechnology: an evidence-based analysis.Ont Health Technol Assess Ser. 2006;6(19):1-43. Epub 2006 Nov 1. Ont Health Technol Assess Ser. 2006. PMID: 23074489 Free PMC article.
-
Nanoparticles in magnetic resonance imaging: from simple to dual contrast agents.Int J Nanomedicine. 2015 Mar 6;10:1727-41. doi: 10.2147/IJN.S76501. eCollection 2015. Int J Nanomedicine. 2015. PMID: 25834422 Free PMC article. Review.
Cited by
-
Effect of lanthanide complex structure on cell viability and association.Inorg Chem. 2014 Jun 16;53(12):6013-21. doi: 10.1021/ic500282n. Epub 2014 Jun 5. Inorg Chem. 2014. PMID: 24901440 Free PMC article.
-
Overcoming the concentration-dependence of responsive probes for magnetic resonance imaging.Metallomics. 2015 Mar;7(3):405-21. doi: 10.1039/c4mt00289j. Metallomics. 2015. PMID: 25579206 Free PMC article. Review.
-
Aqueous Lanthanide Chemistry in Asymmetric Catalysis and Magnetic Resonance Imaging.Synlett. 2016 Jun;27(9):1310-1317. doi: 10.1055/s-0035-1561363. Synlett. 2016. PMID: 27493448 Free PMC article.
-
Recent advances in molecular magnetic resonance imaging of liver fibrosis.Biomed Res Int. 2015;2015:595467. doi: 10.1155/2015/595467. Epub 2015 Mar 22. Biomed Res Int. 2015. PMID: 25874221 Free PMC article. Review.
-
Decoration of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) with N-oxides increases the T1 relaxivity of Gd-complexes.ChemistryOpen. 2024 Jul;13(7):e202300298. doi: 10.1002/open.202300298. Epub 2024 Jan 15. ChemistryOpen. 2024. PMID: 38224205 Free PMC article.
References
-
- Rahmim A, Zaidi H. PET versus SPECT: strength, limitations and challenges. Nucl Med Commun. 2008;29(3):193–207. - PubMed
-
- Filippucci E, Iagnocco A, Meenagh G, et al. Ultrasound imaging for the rheumatologist. Clin Exp Rheumatol. 2006;24:1–5. - PubMed
-
- Morawski AM, Lanza GA, Wickline SA. Targeted contrast agents for magnetic resonance imaging and ultrasound. Curr Opin Biotechnol. 2005;16:89–92. - PubMed
-
- Ntziachristos V. Going deeper than microscopy: the optical imaging frontier in biology. Nat Methods. 2010;7(8):603–614. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
