Stress induces AMPK-dependent loss of potency factors Id2 and Cdx2 in early embryos and stem cells [corrected]
- PMID: 23316940
- PMCID: PMC3653377
- DOI: 10.1089/scd.2012.0352
Stress induces AMPK-dependent loss of potency factors Id2 and Cdx2 in early embryos and stem cells [corrected]
Erratum in
- Stem Cells Dev. 2014 Oct 1;23(19):2401
Abstract
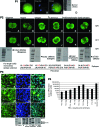
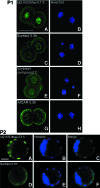
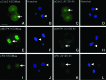
The AMP-activated protein kinase (AMPK) mediates rapid, stress-induced loss of the inhibitor of differentiation (Id)2 in blastocysts and trophoblast stem cells (TSC), and a lasting differentiation in TSC. However, it is not known if AMPK regulates other potency factors or regulates them before the blastocyst stage. The caudal-related homeodomain protein (Cdx)2 is a regulatory gene for determining TSC, the earliest placental lineage in the preimplantation mouse embryo, but is expressed in the oocyte and in early cleavage stage embryos before TSC arise. We assayed the expression of putative potency-maintaining phosphorylated Cdx2 ser60 in the oocyte, two-cell stage embryo, blastocyst, and in TSC. We studied the loss of Cdx2 phospho ser60 expression induced by hyperosmolar stress and its underlying mechanisms. Hyperosmolar stress caused rapid loss of nuclear Cdx2 phospho ser60 and Id2 in the two-cell stage embryo by 0.5 h. Stress-induced Cdx2 phospho ser60 and Id2 loss is reversed by the AMPK inhibitor compound C and is induced by the AMPK agonist 5-amino-1-β-d-ribofuranosyl-imidazole-4-carboxamide in the absence of stress. In the two-cell stage embryo and TSC hyperosmolar, stress caused AMPK-mediated loss of Cdx2 phospho ser60 as detected by immunofluorescence and immunoblot. We propose that AMPK may be the master regulatory enzyme for mediating stress-induced loss of potency as AMPK is also required for stress-induced loss of Id2 in blastocysts and TSC. Since AMPK mediates potency loss in embryos and stem cells it will be important to measure, test mechanisms for, and manage the AMPK function to optimize the stem cell and embryo quality in vitro and in vivo.
Figures



Similar articles
-
Two-cell embryos are more sensitive than blastocysts to AMPK-dependent suppression of anabolism and stemness by commonly used fertility drugs, a diet supplement, and stress.J Assist Reprod Genet. 2017 Dec;34(12):1609-1617. doi: 10.1007/s10815-017-1028-x. Epub 2017 Sep 15. J Assist Reprod Genet. 2017. PMID: 28913567 Free PMC article.
-
Treatment with AICAR inhibits blastocyst development, trophectoderm differentiation and tight junction formation and function in mice.Mol Hum Reprod. 2017 Nov 1;23(11):771-785. doi: 10.1093/molehr/gax050. Mol Hum Reprod. 2017. PMID: 28962017 Free PMC article.
-
Cellular stress causes reversible, PRKAA1/2-, and proteasome-dependent ID2 protein loss in trophoblast stem cells.Reproduction. 2010 Dec;140(6):921-30. doi: 10.1530/REP-10-0268. Epub 2010 Sep 28. Reproduction. 2010. PMID: 20876741 Free PMC article.
-
Why AMPK agonists not known to be stressors may surprisingly contribute to miscarriage or hinder IVF/ART.J Assist Reprod Genet. 2018 Aug;35(8):1359-1366. doi: 10.1007/s10815-018-1213-6. Epub 2018 Jun 7. J Assist Reprod Genet. 2018. PMID: 29882092 Free PMC article. Review.
-
Molecular biology of the stress response in the early embryo and its stem cells.Adv Exp Med Biol. 2015;843:77-128. doi: 10.1007/978-1-4939-2480-6_4. Adv Exp Med Biol. 2015. PMID: 25956296 Review.
Cited by
-
Follistatin supplementation during in vitro embryo culture improves developmental competence of bovine embryos produced using sex-sorted semen.Reprod Biol. 2018 Sep;18(3):267-273. doi: 10.1016/j.repbio.2018.06.004. Epub 2018 Jun 29. Reprod Biol. 2018. PMID: 30196810 Free PMC article.
-
Novel kinetic and developmental transcriptomic pan-stress responses by embryonic stem cells.Sci Rep. 2025 Jul 1;15(1):21291. doi: 10.1038/s41598-025-06628-z. Sci Rep. 2025. PMID: 40594963 Free PMC article.
-
Development and Validation of a Rex1-RFP Potency Activity Reporter Assay That Quantifies Stress-Forced Potency Loss in Mouse Embryonic Stem Cells.Stem Cells Dev. 2016 Feb 15;25(4):320-8. doi: 10.1089/scd.2015.0169. Epub 2016 Jan 29. Stem Cells Dev. 2016. PMID: 26651054 Free PMC article.
-
Using stem cell oxygen physiology to optimize blastocyst culture while minimizing hypoxic stress.J Assist Reprod Genet. 2017 Oct;34(10):1251-1259. doi: 10.1007/s10815-017-0971-x. Epub 2017 Jun 24. J Assist Reprod Genet. 2017. PMID: 28647787 Free PMC article.
-
Prenatal transportation stress alters genome-wide DNA methylation in suckling Brahman bull calves.J Anim Sci. 2018 Dec 3;96(12):5075-5099. doi: 10.1093/jas/sky350. J Anim Sci. 2018. PMID: 30165450 Free PMC article.
References
-
- LaRosa C. Downs SM. Stress stimulates AMP-activated protein kinase and meiotic resumption in mouse oocytes. Biol Reprod. 2006;74:585–592. - PubMed
-
- Xie Y. Awonuga AO. Zhou S. Puscheck EE. Rappolee DA. Interpreting the stress response of early mammalian embryos and their stem cells. Int Rev Cell Mol Biol. 2011;287:43–95. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

