Endoplasmic reticulum and the unfolded protein response: dynamics and metabolic integration
- PMID: 23317820
- PMCID: PMC3666557
- DOI: 10.1016/B978-0-12-407704-1.00005-1
Endoplasmic reticulum and the unfolded protein response: dynamics and metabolic integration
Abstract
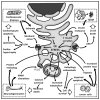
The endoplasmic reticulum (ER) is a dynamic intracellular organelle with multiple functions essential for cellular homeostasis, development, and stress responsiveness. In response to cellular stress, a well-established signaling cascade, the unfolded protein response (UPR), is activated. This intricate mechanism is an important means of re-establishing cellular homeostasis and alleviating the inciting stress. Now, emerging evidence has demonstrated that the UPR influences cellular metabolism through diverse mechanisms, including calcium and lipid transfer, raising the prospect of involvement of these processes in the pathogenesis of disease, including neurodegeneration, cancer, diabetes mellitus and cardiovascular disease. Here, we review the distinct functions of the ER and UPR from a metabolic point of view, highlighting their association with prevalent pathologies.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
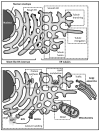
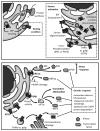
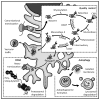
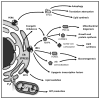
References
-
- Anderson DJ, Hetzer MW. Nuclear envelope formation by chromatin-mediated reorganization of the endoplasmic reticulum. Nat Cell Biol. 2007;9:1160–1166. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

