Safety and effectiveness of HAART in tuberculosis-HIV co-infected patients in Brazil
- PMID: 23317954
- PMCID: PMC3713776
- DOI: 10.5588/ijtld.11.0831
Safety and effectiveness of HAART in tuberculosis-HIV co-infected patients in Brazil
Abstract
Background: Antiretroviral therapy (ART) significantly reduces tuberculosis (TB) incidence among persons with human immunodeficiency virus (HIV), but the safety and effectiveness of concomitant treatment for both diseases remain unclear.
Objective: To evaluate the impact of ART and anti-tuberculosis treatment on survival and risk of adverse events (AE) among co-infected individuals.
Methods: In a retrospective cohort study, clinical data were collected from 618 TB-HIV patients treated with rifampin, isoniazid and pyrazinamide ± ethambutol between 1 January 1995 and 31 December 2003. Patients were categorized into two groups: highly active ART (HAART) or no ART. Different HAART regimens were evaluated. Bivariate analysis, multivariate logistic regression and survival analysis using Cox proportional hazards regression were used.
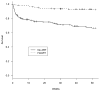
Results: One-year mortality was lower for patients receiving HAART (adjusted hazard ratio [aHR] 0.17, 95%CI 0.09-0.31) compared to no ART. HAART increased the risk of AE (aHR 2.08, 95%CI 1.29-3.36). The odds of AE when receiving a ritonavir + saquinavir HAART regimen was eight-fold higher compared to no ART (OR 8.31, 95%CI 3.04-22.69), while efavirenz-based HAART was not associated with a significantly increased risk of AE (OR 1.42, 95%CI 0.76-2.65).
Conclusion: HIV patients with TB have significantly better survival if they receive HAART during anti-tuberculosis treatment. Efavirenz-based HAART is associated with fewer AEs than protease inhibitor-based HAART.
Conflict of interest statement
Conflict of interest: none declared.
Figures

Similar articles
-
Impact of antiretroviral therapy on the incidence of tuberculosis: the Brazilian experience, 1995-2001.PLoS One. 2007 Sep 5;2(9):e826. doi: 10.1371/journal.pone.0000826. PLoS One. 2007. PMID: 17786198 Free PMC article.
-
Differences in response to antiretroviral therapy in HIV-positive patients being treated for tuberculosis in Eastern Europe, Western Europe and Latin America.BMC Infect Dis. 2018 Apr 23;18(1):191. doi: 10.1186/s12879-018-3077-x. BMC Infect Dis. 2018. PMID: 29685113 Free PMC article.
-
Incidence and predictors of LTFU among adults with TB/HIV co-infection in two governmental hospitals, Mekelle, Ethiopia, 2009-2016: survival model approach.BMC Infect Dis. 2019 Feb 4;19(1):107. doi: 10.1186/s12879-019-3756-2. BMC Infect Dis. 2019. PMID: 30717705 Free PMC article.
-
Integrated therapy for HIV and tuberculosis.AIDS Res Ther. 2016 May 12;13:22. doi: 10.1186/s12981-016-0106-y. eCollection 2016. AIDS Res Ther. 2016. PMID: 27182275 Free PMC article. Review.
-
Tuberculosis associated mortality in a prospective cohort in Sub Saharan Africa: Association with HIV and antiretroviral therapy.Int J Infect Dis. 2017 Mar;56:39-44. doi: 10.1016/j.ijid.2017.01.023. Epub 2017 Feb 1. Int J Infect Dis. 2017. PMID: 28161460 Review.
Cited by
-
Reassuring Birth Outcomes With Tenofovir/Emtricitabine/Efavirenz Used for Prevention of Mother-to-Child Transmission of HIV in Botswana.J Acquir Immune Defic Syndr. 2016 Apr 1;71(4):428-36. doi: 10.1097/QAI.0000000000000847. J Acquir Immune Defic Syndr. 2016. PMID: 26379069 Free PMC article.
-
A Nomogram Model for Mortality Risk Prediction in Pulmonary Tuberculosis Patients Subjected to Directly Observed Treatment Shortcourse (DOTS).Can Respir J. 2022 Dec 16;2022:1449751. doi: 10.1155/2022/1449751. eCollection 2022. Can Respir J. 2022. PMID: 36567966 Free PMC article.
-
Trends of tuberculosis case notification and treatment outcomes in the Sidama Zone, southern Ethiopia: ten-year retrospective trend analysis in urban-rural settings.PLoS One. 2014 Dec 2;9(12):e114225. doi: 10.1371/journal.pone.0114225. eCollection 2014. PLoS One. 2014. PMID: 25460363 Free PMC article.
-
The impact of antiretroviral therapy on mortality in HIV positive people during tuberculosis treatment: a systematic review and meta-analysis.PLoS One. 2014 Nov 12;9(11):e112017. doi: 10.1371/journal.pone.0112017. eCollection 2014. PLoS One. 2014. PMID: 25391135 Free PMC article.
References
-
- World Health Organization. WHO report 2010. Global Tuberculosis Control 2010. WHO/HTM/TB/2010.7. Geneva, Switzerland: WHO; 2011. http://www.doh.state.3.us/disease_ctrl/tb/Trends-Stats/Fact-Sheets/US-Gl....
-
- Santoro-Lopes G, Pinho AMF, Harrison LH, et al. Reduced risk of tuberculosis among Brazilian patients with advanced human immunodefficiency virus treated with highly active anti-retroviral therapy. Clin Infec Dis. 2002;34:543–546. - PubMed
-
- Mukadi YD, Maher D, Harries A. Tuberculosis case fatality rates in high HIV prevalence population in sub-Saharan Africa. AIDS. 2001;13:143–152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

