Economic savings versus health losses: the cost-effectiveness of generic antiretroviral therapy in the United States
- PMID: 23318310
- PMCID: PMC3664029
- DOI: 10.7326/0003-4819-158-2-201301150-00002
Economic savings versus health losses: the cost-effectiveness of generic antiretroviral therapy in the United States
Abstract
Background: U.S. HIV treatment guidelines recommend branded once-daily, 1-pill efavirenz-emtricitabine-tenofovir as first-line antiretroviral therapy (ART). With the anticipated approval of generic efavirenz in the United States, a once-daily, 3-pill alternative (generic efavirenz, generic lamivudine, and tenofovir) will decrease cost but may reduce adherence and virologic suppression.
Objective: To assess the clinical effect, costs, and cost-effectiveness of a 3-pill, generic-based regimen compared with a branded, coformulated regimen and to project the potential national savings in the first year of a switch to generic-based ART.
Design: Mathematical simulation of HIV disease.
Setting: United States.
Patients: HIV-infected persons.
Intervention: No ART (for comparison); 3-pill, generic-based ART; and branded ART.
Measurements: Quality-adjusted life expectancy, costs, and incremental cost-effectiveness ratios (ICERs) in dollars per quality-adjusted life-year (QALY).
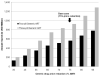
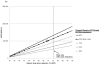
Results: Compared with no ART, generic-based ART has an ICER of $21,100/QALY. Compared with generic-based ART, branded ART increases lifetime costs by $42,500 and per-person survival gains by 0.37 QALYs for an ICER of $114,800/QALY. Estimated first-year savings, if all eligible U.S. patients start or switch to generic-based ART, are $920 million. Most plausible assumptions about generic-based ART efficacy and costs lead to branded ART ICERs greater than $100,000/QALY.
Limitation: The efficacy and price reduction associated with generic drugs are unknown, and estimates are intended to be conservative.
Conclusion: Compared with a slightly less effective generic-based regimen, the cost-effectiveness of first-line branded ART exceeds $100,000/QALY. Generic-based ART in the United States could yield substantial budgetary savings to HIV programs.
Primary funding source: National Institute of Allergy and Infectious Diseases.
Figures



Comment in
-
Generic antiretrovirals and the uncertain future of HIV care in the United States.Ann Intern Med. 2013 Jan 15;158(2):133-4. doi: 10.7326/0003-4819-158-2-201301150-00010. Ann Intern Med. 2013. PMID: 23318314 No abstract available.
-
Cost-effectiveness of generic antiretroviral therapy.Ann Intern Med. 2013 May 21;158(10):776. doi: 10.7326/0003-4819-158-10-201305210-00015. Ann Intern Med. 2013. PMID: 23689769 No abstract available.
-
Cost-effectiveness of generic antiretroviral therapy--in response.Ann Intern Med. 2013 May 21;158(10):776-7. doi: 10.7326/0003-4819-158-10-201305210-00016. Ann Intern Med. 2013. PMID: 23689770 No abstract available.
Similar articles
-
Cost-effectiveness of nucleoside reverse transcriptase inhibitor pairs in efavirenz-based regimens for treatment-naïve adults with HIV infection in the United States.Value Health. 2011 Jul-Aug;14(5):657-64. doi: 10.1016/j.jval.2011.01.009. Epub 2011 Jun 12. Value Health. 2011. PMID: 21839403
-
Comparative Pricing of Branded Tenofovir Alafenamide-Emtricitabine Relative to Generic Tenofovir Disoproxil Fumarate-Emtricitabine for HIV Preexposure Prophylaxis: A Cost-Effectiveness Analysis.Ann Intern Med. 2020 May 5;172(9):583-590. doi: 10.7326/M19-3478. Epub 2020 Mar 10. Ann Intern Med. 2020. PMID: 32150602 Free PMC article.
-
Cost-effectiveness analysis of emtricitabine/tenofovir versus lamivudine/zidovudine, in combination with efavirenz, in antiretroviral-naive, HIV-1-infected patients.Clin Ther. 2008 Feb;30(2):372-81. doi: 10.1016/j.clinthera.2008.02.009. Clin Ther. 2008. PMID: 18343275
-
Efavirenz/emtricitabine/tenofovir disoproxil fumarate single-tablet regimen (Atripla®): a review of its use in the management of HIV infection.Drugs. 2010 Dec 3;70(17):2315-38. doi: 10.2165/11203800-000000000-00000. Drugs. 2010. PMID: 21080746 Review.
-
Single-tablet regimens in HIV: does it really make a difference?Curr Med Res Opin. 2014 Jan;30(1):89-97. doi: 10.1185/03007995.2013.844685. Epub 2013 Oct 4. Curr Med Res Opin. 2014. PMID: 24040862 Review.
Cited by
-
A meta-analysis comparing 48-week treatment outcomes of single and multi-tablet antiretroviral regimens for the treatment of people living with HIV.AIDS Res Ther. 2018 Oct 30;15(1):17. doi: 10.1186/s12981-018-0204-0. AIDS Res Ther. 2018. PMID: 30373620 Free PMC article.
-
Practical considerations in gene therapy for HIV cure.Curr HIV/AIDS Rep. 2014 Mar;11(1):11-9. doi: 10.1007/s11904-013-0197-1. Curr HIV/AIDS Rep. 2014. PMID: 24449226 Free PMC article. Review.
-
Clinical and Economic Impact of Ibalizumab for People With Multidrug-Resistant HIV in the United States.J Acquir Immune Defic Syndr. 2020 Feb 1;83(2):148-156. doi: 10.1097/QAI.0000000000002241. J Acquir Immune Defic Syndr. 2020. PMID: 31929403 Free PMC article.
-
Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: A meta-analysis of randomized controlled trials.Clin Infect Dis. 2014 May;58(9):1297-307. doi: 10.1093/cid/ciu046. Epub 2014 Jan 22. Clin Infect Dis. 2014. PMID: 24457345 Free PMC article.
-
Modeling the cost-effectiveness of HIV treatment: how to buy the most 'health' when resources are limited.Curr Opin HIV AIDS. 2013 Nov;8(6):544-9. doi: 10.1097/COH.0000000000000005. Curr Opin HIV AIDS. 2013. PMID: 24100874 Free PMC article. Review.
References
-
- Thompson MA, Mugavero MJ, Amico KR, Cargill VA, Chang LW, Gross R, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: Evidence-based recommendations from an International Association of Physicians in AIDS Care PaneI. Ann Intern Med. 2012;156(11):817–33. - PMC - PubMed
-
- Schinazi RE. Assessment of the relative potency of emtricitabine and lamivudine. J Acquir Immune Defic Syndr. 2003;34(2):243–5. - PubMed
-
- Svicher V, Alteri C, Artese A, Forbici F, Santoro MM, Schols D, et al. Different evolution of genotypic resistance profiles to emtricitabine versus lamivudine in tenofovir-containing regimens. J Acquir Immune Defic Syndr. 2010;55(3):336–44. - PubMed
-
- Freedberg K, Losina E, Weinstein M, Paltiel A, Cohen C, Seage G, et al. The cost-effectiveness of combination antiretroviral therapy for HIV Disease. N Engl J Med. 2001;244(11):824–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
