Neuroprotective efficacy and pharmacokinetic behavior of novel anti-inflammatory para-phenyl substituted diindolylmethanes in a mouse model of Parkinson's disease
- PMID: 23318470
- PMCID: PMC6067390
- DOI: 10.1124/jpet.112.201558
Neuroprotective efficacy and pharmacokinetic behavior of novel anti-inflammatory para-phenyl substituted diindolylmethanes in a mouse model of Parkinson's disease
Erratum in
-
Correction to "Neuroprotective Efficacy and Pharmacokinetic Behavior of Novel Anti-Inflammatory Para-Phenyl Substituted Diindolylmethanes in a Mouse Model of Parkinson's Disease".J Pharmacol Exp Ther. 2019 Apr;369(1):66. doi: 10.1124/jpet.112.201558err. J Pharmacol Exp Ther. 2019. PMID: 30837300 Free PMC article. No abstract available.
Abstract
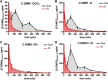
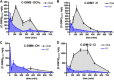
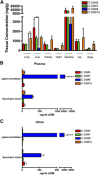
There are currently no registered drugs that slow the progression of neurodegenerative diseases, in part because translation from animal models to the clinic has been hampered by poor distribution to the brain. The present studies examined a selected series of para-phenyl-substituted diindolylmethane (C-DIM) compounds that display anti-inflammatory and neuroprotective efficacy in vitro. We postulated that the pharmacokinetic behavior of C-DIM compounds after oral administration would correlate with neuroprotective efficacy in vivo in a mouse model of Parkinson's disease. Pharmacokinetics and metabolism of 1,1-bis(3'-indolyl)-1-(p-methoxyphenyl)methane (C-DIM5), 1,1-bis(3'-indolyl)-1-(phenyl)methane, 1,1-bis(3'-indolyl)-1-(p-hydroxyphenyl)methane (C-DIM8), and 1,1-bis(3'-indolyl)-1-(p-chlorophenyl)methane (C-DIM12) were determined in plasma and brain of C57Bl/6 mice after oral and intravenous administration at 10 and 1 mg/Kg, respectively. Putative metabolites were measured in plasma, liver, and urine. C-DIM compounds given orally displayed the highest area under the curve, Cmax, and Tmax levels, and C-DIM12 exhibited the most favorable pharmacokinetics of the compounds tested. Oral bioavailability of each compound ranged from 6% (C-DIM8) to 42% (C-DIM12). After pharmacokinetic studies, the neuroprotective efficacy of C-DIM5, C-DIM8, and C-DIM12 (50 mg/Kg per oral) was examined in mice exposed to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and probenecid for 14 days, a model of progressive neurodegeneration with a strong neuroinflammatory component. C-DIM5 and C-DIM12 given orally once daily after one week of exposure to MPTP and probenecid prevented further loss of dopaminergic neurons in the substantia nigra pars compacta and striatal dopamine terminals, indicating that these compounds could be effective therapeutic agents to prevent neurodegeneration.
Figures



Similar articles
-
The Nurr1 Ligand,1,1-bis(3'-Indolyl)-1-(p-Chlorophenyl)Methane, Modulates Glial Reactivity and Is Neuroprotective in MPTP-Induced Parkinsonism.J Pharmacol Exp Ther. 2018 Jun;365(3):636-651. doi: 10.1124/jpet.117.246389. Epub 2018 Apr 6. J Pharmacol Exp Ther. 2018. PMID: 29626009 Free PMC article.
-
Novel para-phenyl substituted diindolylmethanes protect against MPTP neurotoxicity and suppress glial activation in a mouse model of Parkinson's disease.Toxicol Sci. 2015 Feb;143(2):360-73. doi: 10.1093/toxsci/kfu236. Epub 2014 Nov 17. Toxicol Sci. 2015. PMID: 25406165 Free PMC article.
-
Tanshinone I selectively suppresses pro-inflammatory genes expression in activated microglia and prevents nigrostriatal dopaminergic neurodegeneration in a mouse model of Parkinson's disease.J Ethnopharmacol. 2015 Apr 22;164:247-55. doi: 10.1016/j.jep.2015.01.042. Epub 2015 Feb 7. J Ethnopharmacol. 2015. PMID: 25666429
-
Anti-inflammatory and neuroprotective effects of an orally active apocynin derivative in pre-clinical models of Parkinson's disease.J Neuroinflammation. 2012 Oct 23;9:241. doi: 10.1186/1742-2094-9-241. J Neuroinflammation. 2012. PMID: 23092448 Free PMC article.
-
M30, a brain permeable multitarget neurorestorative drug in post nigrostriatal dopamine neuron lesion of parkinsonism animal models.Parkinsonism Relat Disord. 2012 Jan;18 Suppl 1:S151-4. doi: 10.1016/S1353-8020(11)70047-5. Parkinsonism Relat Disord. 2012. PMID: 22166418 Review.
Cited by
-
Polymeric nanoparticles for dopamine and levodopa replacement in Parkinson's disease.Nanoscale Adv. 2022 Nov 3;4(24):5233-5244. doi: 10.1039/d2na00524g. eCollection 2022 Dec 6. Nanoscale Adv. 2022. PMID: 36540116 Free PMC article. Review.
-
Molecular Insights into NR4A2(Nurr1): an Emerging Target for Neuroprotective Therapy Against Neuroinflammation and Neuronal Cell Death.Mol Neurobiol. 2019 Aug;56(8):5799-5814. doi: 10.1007/s12035-019-1487-4. Epub 2019 Jan 25. Mol Neurobiol. 2019. PMID: 30684217 Review.
-
Microbiota, Tryptophan and Aryl Hydrocarbon Receptors as the Target Triad in Parkinson's Disease-A Narrative Review.Int J Mol Sci. 2024 Mar 2;25(5):2915. doi: 10.3390/ijms25052915. Int J Mol Sci. 2024. PMID: 38474162 Free PMC article. Review.
-
A Comprehensive Review on Repurposing the Nanocarriers for the Treatment of Parkinson's Disease: An Updated Patent and Clinical Trials.CNS Neurol Disord Drug Targets. 2025;24(3):181-195. doi: 10.2174/0118715273323074241001071645. CNS Neurol Disord Drug Targets. 2025. PMID: 39400019 Review.
-
Orphan nuclear receptor 4A1 (NR4A1) and novel ligands.Essays Biochem. 2021 Dec 17;65(6):877-886. doi: 10.1042/EBC20200164. Essays Biochem. 2021. PMID: 34096590 Free PMC article.
References
-
- Anderton MJ, Manson MM, Verschoyle R, Gescher A, Steward WP, Williams ML, Mager DE. (2004) Physiological modeling of formulated and crystalline 3,3′-diindolylmethane pharmacokinetics following oral administration in mice. Drug Metab Dispos 32:632–638. - PubMed
-
- Azzaro AJ, Ziemniak J, Kemper E, Campbell BJ, VanDenBerg C. (2007) Pharmacokinetics and absolute bioavailability of selegiline following treatment of healthy subjects with the selegiline transdermal system (6 mg/24 h): a comparison with oral selegiline capsules. J Clin Pharmacol 47:1256–1267. - PubMed
-
- Brown GC, Neher JJ. (2010) Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol 41:242–247. - PubMed
-
- Carta AR, Frau L, Pisanu A, Wardas J, Spiga S, Carboni E. (2011) Rosiglitazone decreases peroxisome proliferator receptor-γ levels in microglia and inhibits TNF-α production: new evidences on neuroprotection in a progressive Parkinson’s disease model. Neuroscience 194:250–261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

