pbx is required for pole and eye regeneration in planarians
- PMID: 23318641
- PMCID: PMC3557772
- DOI: 10.1242/dev.083741
pbx is required for pole and eye regeneration in planarians
Abstract
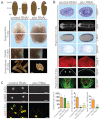
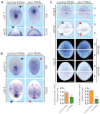
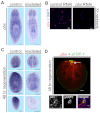
Planarian regeneration involves regionalized gene expression that specifies the body plan. After amputation, planarians are capable of regenerating new anterior and posterior poles, as well as tissues polarized along the anterior-posterior, dorsal-ventral and medial-lateral axes. Wnt and several Hox genes are expressed at the posterior pole, whereas Wnt inhibitory genes, Fgf inhibitory genes, and prep, which encodes a TALE-family homeodomain protein, are expressed at the anterior pole. We found that Smed-pbx (pbx for short), which encodes a second planarian TALE-family homeodomain transcription factor, is required for restored expression of these genes at anterior and posterior poles during regeneration. Moreover, pbx(RNAi) animals gradually lose pole gene expression during homeostasis. By contrast, pbx was not required for initial anterior-posterior polarized responses to wounds, indicating that pbx is required after wound responses for development and maintenance of poles during regeneration and homeostatic tissue turnover. Independently of the requirement for pbx in pole regeneration, pbx is required for eye precursor formation and, consequently, eye regeneration and eye replacement in homeostasis. Together, these data indicate that pbx promotes pole formation of body axes and formation of regenerative progenitors for eyes.
Figures




References
-
- Aboobaker A. A. (2011). Planarian stem cells: a simple paradigm for regeneration. Trends Cell Biol. 21, 304-311 - PubMed
-
- Adell T., Salò E., Boutros M., Bartscherer K. (2009). Smed-Evi/Wntless is required for beta-catenin-dependent and -independent processes during planarian regeneration. Development 136, 905-910 - PubMed
-
- Almuedo-Castillo M., Sureda-Gomez M., Adell T. (2012). Wnt signaling in planarians: new answers to old questions. Dev. Biol. 56, 53-65 - PubMed
-
- Arata Y., Kouike H., Zhang Y., Herman M. A., Okano H., Sawa H. (2006). Wnt signaling and a Hox protein cooperatively regulate psa-3/Meis to determine daughter cell fate after asymmetric cell division in C. elegans. Dev. Cell 11, 105-115 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

