S1pr2/Gα13 signaling controls myocardial migration by regulating endoderm convergence
- PMID: 23318642
- PMCID: PMC3557776
- DOI: 10.1242/dev.085340
S1pr2/Gα13 signaling controls myocardial migration by regulating endoderm convergence
Abstract
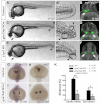
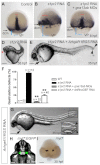
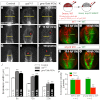
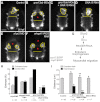
A key process during vertebrate heart development is the migration of bilateral populations of myocardial precursors towards the midline to form the primitive heart tube. In zebrafish, signaling mediated by sphingosine-1-phosphate (S1P) and its cognate G protein-coupled receptor (S1pr2/Mil) is essential for myocardial migration, but the underlying mechanisms remain undefined. Here, we show that suppression of Gα(13) signaling disrupts myocardial migration, leading to the formation of two bilaterally located hearts (cardia bifida). Genetic studies indicate that Gα(13) acts downstream of S1pr2 to regulate myocardial migration through a RhoGEF-dependent pathway. Furthermore, disrupting any component of the S1pr2/Gα(13)/RhoGEF pathway impairs endoderm convergence during segmentation, and the endodermal defects correlate with the extent of cardia bifida. Moreover, endoderm transplantation reveals that the presence of wild-type anterior endodermal cells in Gα(13)-deficient embryos is sufficient to rescue the endoderm convergence defect and cardia bifida, and, conversely, that the presence of anterior endodermal cells defective for S1pr2 or Gα(13) in wild-type embryos causes such defects. Thus, S1pr2/Gα(13) signaling probably acts in the endoderm to regulate myocardial migration. In support of this notion, cardiac-specific expression of Gα(13) fails to rescue cardia bifida in the context of global Gα(13) inhibition. Our data demonstrate for the first time that the Gα(13)/RhoGEF-dependent pathway functions downstream of S1pr2 to regulate convergent movement of the endoderm, an event that is crucial for coordinating myocardial migration.
Figures



References
-
- Alexander J., Rothenberg M., Henry G. L., Stainier D. Y. (1999). casanova plays an early and essential role in endoderm formation in zebrafish. Dev. Biol. 215, 343-357 - PubMed
-
- Alsan B. H., Schultheiss T. M. (2002). Regulation of avian cardiogenesis by Fgf8 signaling. Development 129, 1935-1943 - PubMed
-
- Ancellin N., Hla T. (1999). Differential pharmacological properties and signal transduction of the sphingosine 1-phosphate receptors EDG-1, EDG-3, and EDG-5. J. Biol. Chem. 274, 18997-19002 - PubMed
-
- Andree B., Duprez D., Vorbusch B., Arnold H. H., Brand T. (1998). BMP-2 induces ectopic expression of cardiac lineage markers and interferes with somite formation in chicken embryos. Mech. Dev. 70, 119-131 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

