Synthesis of N3-substituted carboranyl thymidine bioconjugates and their evaluation as substrates of recombinant human thymidine kinase 1
- PMID: 23318906
- PMCID: PMC3587680
- DOI: 10.1016/j.ejmech.2012.11.041
Synthesis of N3-substituted carboranyl thymidine bioconjugates and their evaluation as substrates of recombinant human thymidine kinase 1
Abstract
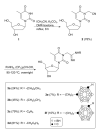
Four different libraries of overall twenty three N3-substituted thymidine (dThd) analogues, including eleven 3-carboranyl thymidine analogues (3CTAs), were synthesized. The latter are potential agents for Boron Neutron Capture Therapy (BNCT) of cancer. Linker between the dThd scaffold and the m-carborane cluster at the N3-position of the 3CTAs contained amidinyl-(3e and 3f), guanidyl-(7e-7g), tetrazolylmethyl-(9b1/2-9d1/2), or tetrazolyl groups (11b1/2-11d1/2) to improve human thymidine kinase 1 (hTK1) substrate characteristics and water solubilities compared with 1st generation 3CTAs, such as N5 and N5-2OH. The amidinyl- and guanidyl-type N3-substitued dThd analogues (3a-3f and 7a-7g) had hTK1 phosphorylation rates of <30% relative to that of dThd, the endogenous hTK1 substrate, whereas the tetrazolyl-type N3-substitued dThd analogues (9a, 9b1/2-9d1/2 and 11a, 11b1/2-11d1/2) had relative phosphorylation rates (rPRs) of >40%. Compounds 9a, 9b1/2-9d1/2 and 11a, 11b1/2-11d1/2 were subjected to in-depth enzyme kinetics studies and the obtained rk(cat)/K(m) (k(cat)/K(m) relative to that of dThd) ranged from 2.5 to 26%. The tetrazolyl-type N3-substitued dThd analogues 9b1/2 and 11d1/2 were the best substrates of hTK1 with rPRs of 52.4% and 42.5% and rk(cat)/K(m) values of 14.9% and 19.7% respectively. In comparison, the rPR and rk(cat)/K(m) values of N5-2OH in this specific study were 41.5% and 10.8%, respectively. Compounds 3e and 3f were >1900 and >1500 times, respectively, better soluble in PBS (pH 7.4) than N5-2OH whereas solubilities for 9b1/2-9d1/2 and 11b1/2-11d1/2 were only 1.3-13 times better.
Copyright © 2012 Elsevier Masson SAS. All rights reserved.
Figures





References
-
- Tjarks W, Tiwari R, Byun Y, Narayanasamy S, Barth RF. Carboranyl thymidine analouges for neutron capture therapy. Chem. Commun. 2007:4978–4991. - PubMed
-
- Soloway AH, Tjarks W, Barnum BA, Rong F–G, Barth RF, Codogni IM, Wilson JG. The chemistry of neutron capture therapy. Chem. Rev. 1998;98:1515–1562. - PubMed
-
- Sherley JL, Kelly TJ. Human cytosolic thymidine kinase. Purification and physical characterization of the enzyme from HeLa cells. J. Biol. Chem. 1988;263:375–382. - PubMed
-
- Shields AF, Grierson JR, Dohmen BM, Machulla HJ, Stayanoff JC, Crews J. M. Lawhorn, Obradovich JE, Muzik1 O, Mangner TJ. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat. Med. 1998;4:1334–1336. - PubMed
-
- Byun Y, Thirumamagal BTS, Yang W, Eriksson S, Barth RF, Tjarks W. Preparation and biological evaluationof 10B-Enriched 3-[5-{2-(2,3-dihydroxyprop-1-yl)-o-carboran-1-yl}pentan-1-yl]thymidine (N5-2OH) J. Med. Chem. 2006;49:5513–5523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

