Focal Scn1a knockdown induces cognitive impairment without seizures
- PMID: 23318929
- PMCID: PMC3628954
- DOI: 10.1016/j.nbd.2012.12.021
Focal Scn1a knockdown induces cognitive impairment without seizures
Abstract
Cognitive impairment is a common comorbidity in pediatric epilepsy that can severely affect quality of life. In many cases, antiepileptic treatments fail to improve cognition. Therefore, a fundamental question is whether underlying brain abnormalities may contribute to cognitive impairment through mechanisms independent of seizures. Here, we examined the possible effects on cognition of Nav1.1 down-regulation, a sodium channel principally involved in Dravet syndrome but also implicated in other cognitive disorders, including autism and Alzheimer's disease. Using an siRNA approach to knockdown Nav1.1 selectively in the basal forebrain region, we were able to target a learning and memory network while avoiding the generation of spontaneous seizures. We show that reduction of Nav1.1 expression in the medial septum and diagonal band of Broca leads to a dysregulation of hippocampal oscillations in association with a spatial memory deficit. We propose that the underlying etiology responsible for Dravet syndrome may directly contribute to cognitive impairment in a manner that is independent from seizures.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


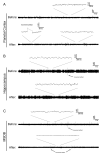
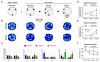
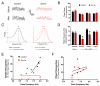
References
-
- Akiyama M, Kobayashi K, Yoshinaga H, Ohtsuka Y. A long-term follow-up study of Dravet syndrome up to adulthood. Epilepsia. 2010;51:1043–1052. - PubMed
-
- Ben-ari Y, Holmes GL. Effects of seizures on developmental processes in the immature brain. Lancet Neurology. 2006;5:1055–1063. - PubMed
-
- Bland BH, Oddie SD. Theta band oscillation and synchrony in the hippocampal formation and associated structures: the case for its role in sensorimotor integration. Behavioural Brain Research. 2001;127:119–136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS076763/NS/NINDS NIH HHS/United States
- R01 NS074450/NS/NINDS NIH HHS/United States
- R01 NS073083/NS/NINDS NIH HHS/United States
- NS074450/NS/NINDS NIH HHS/United States
- MH086833/MH/NIMH NIH HHS/United States
- R21 MH086833/MH/NIMH NIH HHS/United States
- NS073083/NS/NINDS NIH HHS/United States
- F31NS077537-01/NS/NINDS NIH HHS/United States
- R01 NS075249/NS/NINDS NIH HHS/United States
- NS075249/NS/NINDS NIH HHS/United States
- R01 NS076763/NS/NINDS NIH HHS/United States
- F31 NS077537/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

