Myofilament incorporation and contractile function after gene transfer of cardiac troponin I Ser43/45Ala
- PMID: 23318976
- PMCID: PMC3640666
- DOI: 10.1016/j.abb.2012.12.021
Myofilament incorporation and contractile function after gene transfer of cardiac troponin I Ser43/45Ala
Abstract
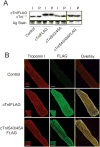
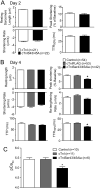
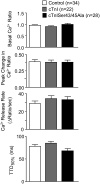
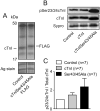
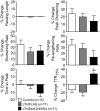
Phosphorylation of cardiac troponin I serines 43/45 (cTnISer43/45) by protein kinase C (PKC) is associated with cardiac dysfunction and yet there is disagreement about the role this cluster plays in modulating contractile performance. The present study evaluates the impact of phospho-null Ala substitutions at Ser43/45 (cTnISer43/45Ala) on contractile performance in intact myocytes. Viral-based gene transfer of cardiac troponin I (cTnI) or cTnISer43/45Ala resulted in time-dependent increases in expression, with 70-80% of endogenous cTnI replaced within 4days. Western analysis of intact and permeabilized myocytes along with immunohistochemistry showed each exogenous cTnI was incorporated into the sarcomere of myocytes. In contractile function studies, there were no differences in shortening and re-lengthening for cTnI and cTnISer43/45Ala-expressing myocytes 2days after gene transfer. However, more extensive replacement with cTnISer43/45Ala after 4days diminished peak shortening amplitude and accelerated re-lengthening measured as the time to 50% re-lengthening (TTR50%). A decrease in myofilament Ca(2+) sensitivity of tension also was observed in permeabilized myocytes expressing cTnISer43/45Ala and is consistent with accelerated re-lengthening observed in intact myocytes under basal conditions. Phosphorylation of cTnI Ser23/24 and the Ca(2+) transient were not changed in these myocytes. These results demonstrate extensive sarcomere expression of cTnISer43/45Ala directly modulates myofilament function under basal conditions. In further work, the accelerated re-lengthening observed in control or cTnI-expressing myocytes treated with the PKC agonist, endothelin-1 (ET, 10nM) was slowed in myocytes expressing cTnISer43/45Ala. This outcome may indicate Ser43/45 is targeted for phosphorylation by ET-activated PKC and/or influences transduction of this agonist-activated response.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
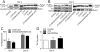
References
-
- Gordon AM, Regnier M, Homsher E. Skeletal and cardiac muscle contractile activation: tropomyosin “rocks and rolls”. News Physiol Sci. 2001;16:49–55. - PubMed
-
- Davis J, et al. Designing heart performance by gene transfer. Physiol Rev. 2008;88(4):1567–651. - PubMed
-
- Noland TA, Jr, Raynor RL, Kuo JF. Identification of sites phosphorylated in bovine cardiac troponin I and troponin T by protein kinase C and comparative substrate activity of synthetic peptides containing the phosphorylation sites. J Biol Chem. 1989;264(34):20778–85. - PubMed
-
- Noguchi T, et al. Thin-filament-based modulation of contractile performance in human heart failure. Circulation. 2004;110(8):982–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

