FOSL1 controls the assembly of endothelial cells into capillary tubes by direct repression of αv and β3 integrin transcription
- PMID: 23319049
- PMCID: PMC3592019
- DOI: 10.1128/MCB.01054-12
FOSL1 controls the assembly of endothelial cells into capillary tubes by direct repression of αv and β3 integrin transcription
Abstract
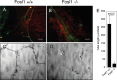
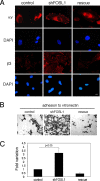
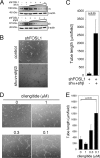
To form three-dimensional capillary tubes, endothelial cells must establish contacts with the extracellular matrix that provides signals for their proliferation, migration, and differentiation. The transcription factor Fosl1 plays a key role in the vasculogenic and angiogenic processes as Fosl1 knockout embryos die with vascular defects in extraembryonic tissues. Here, we show that Fosl1(-/-) embryonic stem cells differentiate into endothelial cells but fail to correctly assemble into primitive capillaries and to form tube-like structures. FOSL1 silencing affects in vitro angiogenesis, increases cell adhesion, and decreases cell mobility of primary human endothelial cells (HUVEC). We further show that FOSL1 is a repressor of αv and β3 integrin expression and that the down-modulation of αvβ3 rescues the angiogenic phenotype in FOSL1-silenced HUVEC, while the ectopic expression of αvβ3 alone reproduces the phenotypic alterations induced by FOSL1 knockdown. FOSL1 represses the transcription of both αv and β3 integrin genes by binding together with JunD to their proximal promoter via the transcription factor SP1. These data suggest that FOSL1-dependent negative regulation of αvβ3 expression on endothelial cells is required for endothelial assembly into vessel structures.
Figures





Similar articles
-
Role of the AP-1 transcription factor FOSL1 in endothelial cells adhesion and migration.Cell Adh Migr. 2013 Sep-Oct;7(5):408-11. doi: 10.4161/cam.25894. Epub 2013 Jul 25. Cell Adh Migr. 2013. PMID: 24084233 Free PMC article. Review.
-
Variable effects of alpha v suppression on VEGFR-2 expression in endothelial cells of different vascular beds.Thromb Haemost. 2009 Nov;102(5):975-82. doi: 10.1160/TH08-10-0687. Thromb Haemost. 2009. PMID: 19888537
-
Deficiency of αII-spectrin affects endothelial cell-matrix contact and migration leading to impairment of angiogenesis in vitro.Cell Mol Biol Lett. 2020 Feb 3;25:3. doi: 10.1186/s11658-020-0200-y. eCollection 2020. Cell Mol Biol Lett. 2020. PMID: 32042281 Free PMC article.
-
Glycation of vitronectin inhibits VEGF-induced angiogenesis by uncoupling VEGF receptor-2-αvβ3 integrin cross-talk.Cell Death Dis. 2015 Jun 25;6(6):e1796. doi: 10.1038/cddis.2015.174. Cell Death Dis. 2015. PMID: 26111058 Free PMC article.
-
Role of the Transcription Factor FOSL1 in Organ Development and Tumorigenesis.Int J Mol Sci. 2022 Jan 28;23(3):1521. doi: 10.3390/ijms23031521. Int J Mol Sci. 2022. PMID: 35163444 Free PMC article. Review.
Cited by
-
FOSL1 is a novel mediator of endotoxin/lipopolysaccharide-induced pulmonary angiogenic signaling.Sci Rep. 2020 Aug 4;10(1):13143. doi: 10.1038/s41598-020-69735-z. Sci Rep. 2020. PMID: 32753701 Free PMC article.
-
A multi-step transcriptional cascade underlies vascular regeneration in vivo.Sci Rep. 2018 Apr 3;8(1):5430. doi: 10.1038/s41598-018-23653-3. Sci Rep. 2018. PMID: 29615716 Free PMC article.
-
Investigating the role of super-enhancer RNAs underlying embryonic stem cell differentiation.BMC Genomics. 2019 Dec 30;20(Suppl 10):896. doi: 10.1186/s12864-019-6293-x. BMC Genomics. 2019. PMID: 31888456 Free PMC article.
-
Comparison of stem/progenitor cell number and transcriptomic profile in the mammary tissue of dairy and beef breed heifers.J Appl Genet. 2014 Aug;55(3):383-95. doi: 10.1007/s13353-014-0213-1. Epub 2014 Apr 20. J Appl Genet. 2014. PMID: 24748329 Free PMC article.
-
FRA1 promotes squamous cell carcinoma growth and metastasis through distinct AKT and c-Jun dependent mechanisms.Oncotarget. 2016 Jun 7;7(23):34371-83. doi: 10.18632/oncotarget.9110. Oncotarget. 2016. PMID: 27144339 Free PMC article.
References
-
- Bader BL, Rayburn H, Crowley D, Hynes RO. 1998. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell 95: 507– 519 - PubMed
-
- Drake CJ, Cheresh DA, Little CD. 1995. An antagonist of integrin alpha v beta 3 prevents maturation of blood vessels during embryonic neovascularization. J. Cell Sci. 108: 2655– 2661 - PubMed
-
- Stupack DG, Cheresh DA. 2004. Integrins and angiogenesis. Curr. Top. Dev. Biol. 64: 207– 238 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
