AAV-mediated overexpression of human α7 integrin leads to histological and functional improvement in dystrophic mice
- PMID: 23319059
- PMCID: PMC3589167
- DOI: 10.1038/mt.2012.281
AAV-mediated overexpression of human α7 integrin leads to histological and functional improvement in dystrophic mice
Abstract
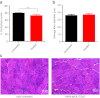
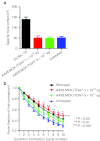
Duchenne muscular dystrophy (DMD) is a severe muscle disease caused by mutations in the DMD gene, with loss of its gene product, dystrophin. Dystrophin helps link integral membrane proteins to the actin cytoskeleton and stabilizes the sarcolemma during muscle activity. We investigated an alternative therapeutic approach to dystrophin replacement by overexpressing human α7 integrin (ITGA7) using adeno-associated virus (AAV) delivery. ITGA7 is a laminin receptor in skeletal and cardiac muscle that links the extracellular matrix (ECM) to the actin skeleton. It is modestly upregulated in DMD muscle and has been proposed to be an important modifier of dystrophic symptoms. We delivered rAAV8.MCK.ITGA7 to the lower limb of mdx mice through isolated limb perfusion (ILP) of the femoral artery. We demonstrated ~50% of fibers in the tibialis anterior (TA) and extensor digitorum longus (EDL) overexpressing α7 integrin at the sarcolemma following AAV gene transfer. The increase in ITGA7 in skeletal muscle significantly protected against loss of force following eccentric contraction-induced injury compared with untreated (contralateral) muscles while specific force following tetanic contraction was unchanged. Reversal of additional dystrophic features included reduced Evans blue dye (EBD) uptake and increased muscle fiber diameter. Taken together, this data shows that rAAV8.MCK.ITGA7 gene transfer stabilizes the sarcolemma potentially preserving mdx muscle from further damage. This therapeutic approach demonstrates promise as a viable treatment for DMD with further implications for other forms of muscular dystrophy.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

