Zinc biochemistry: from a single zinc enzyme to a key element of life
- PMID: 23319127
- PMCID: PMC3648744
- DOI: 10.3945/an.112.003038
Zinc biochemistry: from a single zinc enzyme to a key element of life
Abstract
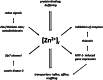
The nutritional essentiality of zinc for the growth of living organisms had been recognized long before zinc biochemistry began with the discovery of zinc in carbonic anhydrase in 1939. Painstaking analytical work then demonstrated the presence of zinc as a catalytic and structural cofactor in a few hundred enzymes. In the 1980s, the field again gained momentum with the new principle of "zinc finger" proteins, in which zinc has structural functions in domains that interact with other biomolecules. Advances in structural biology and a rapid increase in the availability of gene/protein databases now made it possible to predict zinc-binding sites from metal-binding motifs detected in sequences. This procedure resulted in the definition of zinc proteomes and the remarkable estimate that the human genome encodes ∼3000 zinc proteins. More recent developments focus on the regulatory functions of zinc(II) ions in intra- and intercellular information transfer and have tantalizing implications for yet additional functions of zinc in signal transduction and cellular control. At least three dozen proteins homeostatically control the vesicular storage and subcellular distribution of zinc and the concentrations of zinc(II) ions. Novel principles emerge from quantitative investigations on how strongly zinc interacts with proteins and how it is buffered to control the remarkably low cellular and subcellular concentrations of free zinc(II) ions. It is fair to conclude that the impact of zinc for health and disease will be at least as far-reaching as that of iron.
Conflict of interest statement
Author disclosure: W. Maret, no conflicts of interest.
Figures


References
-
- Raulin J. Etudes chimiques sur la vegetation. Ann Sci Nat Bot Biol Veg. 1869;11:92–299
-
- Lutz RE. The normal occurrence of zinc in biologic material. J Ind Hyg. 1926;8:127
-
- Drinker KA, Collier ES. The significance of zinc in the living organism. J Ind Hyg. 1926;8:257
-
- Todd WR, Elvehjem CA, Hart EB. Zinc in the nutrition of the rat. Am J Physiol. 1934;107:146–56
-
- Prasad AS, Halsted JA, Nadimi M. Syndrome of iron deficiency anemia, hepatosplenomegaly, hypogonadism, dwarfism and geophagia. Am J Med. 1961;31:532–46 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

