Functional interplay between the cell cycle and cell phenotypes
- PMID: 23319145
- PMCID: PMC3813296
- DOI: 10.1039/c2ib20246h
Functional interplay between the cell cycle and cell phenotypes
Abstract
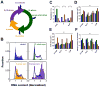
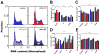
Cell cycle distribution of adherent cells is typically assessed using flow cytometry, which precludes the measurements of many cell properties and their cycle phase in the same environment. Here we develop and validate a microscopy system to quantitatively analyze the cell-cycle phase of thousands of adherent cells and their associated cell properties simultaneously. This assay demonstrates that population-averaged cell phenotypes can be written as a linear combination of cell-cycle fractions and phase-dependent phenotypes. By perturbing the cell cycle through inhibition of cell-cycle regulators or changing nuclear morphology by depletion of structural proteins, our results reveal that cell cycle regulators and structural proteins can significantly interfere with each other's prima facie functions. This study introduces a high-throughput method to simultaneously measure the cell cycle and phenotypes at single-cell resolution, which reveals a complex functional interplay between the cell cycle and cell phenotypes.
Figures




References
-
- Hartwell L, Kastan M. Cell cycle control and cancer. Science. 1994;266:1821–1828. - PubMed
-
- Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273:345–9. - PubMed
-
- van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–2054. - PubMed
-
- Sherr CJ. Cancer Cell Cycles. Science. 1996;274:1672–1677. - PubMed
-
- Wang W, Bu B, Xie M, Zhang M, Yu Z, Tao D. Neural cell cycle dysregulation and central nervous system diseases. Progress in Neurobiology. 2009;89:1–17. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

