Pregnancy represses induction of efflux transporters in livers of type I diabetic mice
- PMID: 23319174
- PMCID: PMC3644534
- DOI: 10.1007/s11095-013-0981-z
Pregnancy represses induction of efflux transporters in livers of type I diabetic mice
Abstract
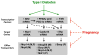
Purpose: To determine whether down-regulation of transcription factor signaling during pregnancy disrupts the induction of efflux transporters in type I diabetic mice.
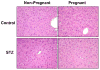
Methods: Type I diabetes was induced in female C57BL/6 mice with multiple low dose intraperitoneal injections of streptozotocin (STZ) at least 2 weeks prior to mating with normoglycemic male mice. On gestation day 14, livers were collected from vehicle- and STZ-treated non-pregnant and pregnant mice for quantification of efflux transporter and transcription factor signaling.
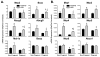
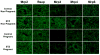
Results: STZ treatment up-regulated expression of Mrp1-5, Mdr1, Abcg5, Abcg8, Bcrp, and Bsep mRNA and/or protein in the livers of non-pregnant mice. Interestingly, little to no change in transporter expression was observed in STZ-treated pregnant mice compared to vehicle- and STZ-treated non-pregnant mice.
Conclusions: This study demonstrates the opposing regulation of hepatobiliary efflux transporters in response to diabetes and pregnancy and points to PPARγ, Nrf2, and FXR as candidate pathways underlying the differential expression of transporters.
Figures

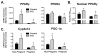
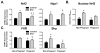
References
-
- Cheng Q, Aleksunes LM, Manautou JE, Cherrington NJ, Scheffer GL, Yamasaki H, et al. Drug-metabolizing enzyme and transporter expression in a mouse model of diabetes and obesity. Mol Pharm. 2008 Jan-Feb;5(1):77–91. - PubMed
-
- Nowicki MT, Aleksunes LM, Sawant SP, Dnyanmote AV, Mehendale HM, Manautou JE. Renal and hepatic transporter expression in type 2 diabetic rats. Drug Metab Lett. 2008 Jan;2(1):11–7. - PubMed
-
- Hasegawa Y, Kishimoto S, Shibatani N, Inotsume N, Takeuchi Y, Fukushima S. The disposition of pravastatin in a rat model of streptozotocin-induced diabetes and organic anion transporting polypeptide 2 and multidrug resistance-associated protein 2 expression in the liver. Biol Pharm Bull. 2010 Jan;33(1):153–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

