Proteomic analysis of novel targets associated with TrkA-mediated tyrosine phosphorylation signaling pathways in SK-N-MC neuroblastoma cells
- PMID: 23319303
- PMCID: PMC3580882
- DOI: 10.1002/pmic.201200251
Proteomic analysis of novel targets associated with TrkA-mediated tyrosine phosphorylation signaling pathways in SK-N-MC neuroblastoma cells
Abstract
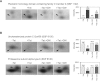
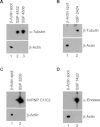
Tropomyosin-related kinase A (TrkA) is a receptor-type protein tyrosine kinase and exploits pleiotypic roles via nerve growth factor (NGF)-dependent or NGF-independent mechanisms in various cell types. Here, we showed that the inhibition of TrkA activity by GW441756 resulted in the suppression of tyrosine phosphorylation of cellular proteins including extracellular signal-regulated protein kinase (ERK) and c-Jun N-terminal kinase (JNK). To find novel targets associated with TrkA-mediated tyrosine phosphorylation signaling pathways, we investigated GW441756 effects on TrkA-dependent targets in SK-N-MC neuroblastoma cells by proteomic analysis. The major TrkA-dependent protein spots controlled by GW441756 were determined by PDQuest image analysis, identified by MALDI-TOF MS and MALDI-TOF/TOF MS/MS, and verified by 2DE/Western blot analysis. Thus, we found that most of the identified protein spots were modified forms in a normal condition, and their modifications were regulated by TrkA activity. Especially, our results demonstrated that the modifications of α-tubulin and heterogeneous nuclear ribonucleoproteins C1/C2 (hnRNP C1/C2) were significantly upregulated by TrkA, whereas α-enolase modification was downregulated by TrkA, and it was suppressed by GW441756, indicating that TrkA activity is required for their modifications. Taken together, we suggest here that the major novel TrkA-dependent targets such as α-tubulin, hnRNP C1/C2, and α-enolase could play an essential role in TrkA-mediated tyrosine phosphorylation signaling pathways via regulation of their posttranslational modifications.
© 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures







Similar articles
-
Proteomic analysis of novel targets associated with the enhancement of TrkA-induced SK-N-MC cancer cell death caused by NGF.Exp Mol Med. 2016 May 27;48(5):e235. doi: 10.1038/emm.2016.33. Exp Mol Med. 2016. PMID: 27229480 Free PMC article.
-
Proteomic analysis of SP600125-controlled TrkA-dependent targets in SK-N-MC neuroblastoma cells: inhibition of TrkA activity by SP600125.Proteomics. 2014 Feb;14(2-3):202-15. doi: 10.1002/pmic.201300023. Proteomics. 2014. PMID: 24375967
-
Constitutively active Src facilitates NGF-induced phosphorylation of TrkA and causes enhancement of the MAPK signaling in SK-N-MC cells.FEBS Lett. 2004 Feb 27;560(1-3):215-20. doi: 10.1016/S0014-5793(04)00115-2. FEBS Lett. 2004. PMID: 14988025
-
Apoptotic cell death in TrkA-overexpressing cells: kinetic regulation of ERK phosphorylation and caspase-7 activation.Mol Cells. 2008 Jul 31;26(1):12-7. Epub 2008 May 20. Mol Cells. 2008. PMID: 18511888
-
Alternative TrkAIII splicing: a potential regulated tumor-promoting switch and therapeutic target in neuroblastoma.Future Oncol. 2005 Oct;1(5):689-98. doi: 10.2217/14796694.1.5.689. Future Oncol. 2005. PMID: 16556046 Review.
Cited by
-
TrkAIII promotes microtubule nucleation and assembly at the centrosome in SH-SY5Y neuroblastoma cells, contributing to an undifferentiated anaplastic phenotype.Biomed Res Int. 2013;2013:740187. doi: 10.1155/2013/740187. Epub 2013 Jun 6. Biomed Res Int. 2013. PMID: 23841091 Free PMC article.
-
Ginsenoside Rg3 Restores Mitochondrial Cardiolipin Homeostasis via GRB2 to Prevent Parkinson's Disease.Adv Sci (Weinh). 2024 Oct;11(39):e2403058. doi: 10.1002/advs.202403058. Epub 2024 Aug 19. Adv Sci (Weinh). 2024. PMID: 39159293 Free PMC article.
-
Huntingtin-associated protein 1 ameliorates neurological function rehabilitation by facilitating neurite elongation through TrKA-MAPK pathway in mice spinal cord injury.Front Mol Neurosci. 2023 Aug 7;16:1214150. doi: 10.3389/fnmol.2023.1214150. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37609072 Free PMC article.
-
Proteomic analysis of novel targets associated with the enhancement of TrkA-induced SK-N-MC cancer cell death caused by NGF.Exp Mol Med. 2016 May 27;48(5):e235. doi: 10.1038/emm.2016.33. Exp Mol Med. 2016. PMID: 27229480 Free PMC article.
References
-
- Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr. Opin. Neurobiol. 2001;11:272–280. - PubMed
-
- Cunningham ME, Stephens RM, Kaplan DR, Greene LA. Autophosphorylation of activation loop tyrosines regulates signaling by the TRK nerve growth factor receptor. J. Biol. Chem. 1997;272:10957–10967. - PubMed
-
- Hallberg B, Ashcroft M, Loeb DM, Kaplan DR, et al. Nerve growth factor induced stimulation of Ras requires Trk interaction with Shc but does not involve phosphoinositide 3-OH kinase. Oncogene. 1998;17:691–697. - PubMed
-
- Tsuruda A, Suzuki S, Maekawa T, Oka S. Constitutively active Src facilitates NGF-induced phosphorylation of TrkA and causes enhancement of the MAPK signaling in SKNMC cells. FEBS Lett. 2004;560:215–220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

