Characterization of a distinct host response profile to Pneumocystis murina asci during clearance of pneumocystis pneumonia
- PMID: 23319554
- PMCID: PMC3584873
- DOI: 10.1128/IAI.01181-12
Characterization of a distinct host response profile to Pneumocystis murina asci during clearance of pneumocystis pneumonia
Abstract
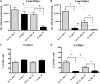
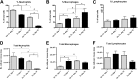
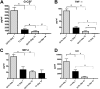
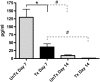
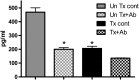
Pneumocystis spp. are yeast-like fungi that cause pneumocystis pneumonia (PcP) in immunocompromised individuals and exacerbate chronic lung diseases in immunocompetent individuals. The Pneumocystis life cycle includes trophic forms and asci (cyst forms). The cell walls of Pneumocystis asci contain β-1,3-D-glucan, and treatment of PcP with β-1,3-D-glucan synthase inhibitors, such as anidulafungin, results in depletion of asci, but not trophic forms. The pulmonary host response during immune reconstitution (IR)-mediated clearance of PcP in anidulafungin-treated and untreated mice was characterized to identify ascus-specific responses. During IR, similar numbers of trophic forms were present in the anidulafungin-treated and untreated mice; however, asci were only present in the untreated mice. IR resulted in a significant reduction of trophic forms from the lungs in both groups and asci in the untreated group. The presence of asci in untreated mice correlated with increased β-glucan content in the lungs. The untreated mice mounted immune responses associated with a deleterious host inflammatory response, including increased CD8(+) T cell influx and expression of macrophage inflammatory response markers. A more robust cellular response was also observed in the untreated mice, with increased numbers of macrophages and neutrophils that were associated with greater lung damage. Markers of a Th17 response were also elevated in the untreated mice. These results suggest that the host mounts unique responses to asci and trophic forms. That these 2 life cycle stages provoked distinct host response profiles has significant implications for clearance and interpretation of the host immune responses to PcP.
Figures



References
-
- Liu J, Balasubramanian MK. 2001. 1,3-Beta glucan synthase: a useful target for antifungal drugs. Curr. Drug Targets Infect. Disord. 1: 159–169 - PubMed
-
- Williams DJ, Radding JA, Dell A, Khoo KH, Rogers ME, Richards FF, Armstrong MY. 1991. Glucan synthesis in Pneumocystis carinii. J. Protozool. 38: 427–437 - PubMed
-
- Kottom TJ, Limper AH. 1999. Assembly of cell wall glucans by Pneumocystis carinii: characterization of the Gsc-1 subunit mediating beta-glucan synthesis. J. Eukaryot. Microbiol. 46(Suppl): 131S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

