BtaE, an adhesin that belongs to the trimeric autotransporter family, is required for full virulence and defines a specific adhesive pole of Brucella suis
- PMID: 23319562
- PMCID: PMC3584859
- DOI: 10.1128/IAI.01241-12
BtaE, an adhesin that belongs to the trimeric autotransporter family, is required for full virulence and defines a specific adhesive pole of Brucella suis
Abstract
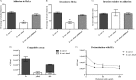
Brucella is responsible for brucellosis, one of the most common zoonoses worldwide that causes important economic losses in several countries. Increasing evidence indicates that adhesion of Brucella spp. to host cells is an important step to establish infection. We have previously shown that the BmaC unipolar monomeric autotransporter mediates the binding of Brucella suis to host cells through cell-associated fibronectin. Our genome analysis shows that the B. suis genome encodes several additional potential adhesins. In this work, we characterized a predicted trimeric autotransporter that we named BtaE. By expressing btaE in a nonadherent Escherichia coli strain and by phenotypic characterization of a B. suis ΔbtaE mutant, we showed that BtaE is involved in the binding of B. suis to hyaluronic acid. The B. suis ΔbtaE mutant exhibited a reduction in the adhesion to HeLa and A549 epithelial cells compared with the wild-type strain, and it was outcompeted by the wild-type strain in the binding to HeLa cells. The knockout btaE mutant showed an attenuated phenotype in the mouse model, indicating that BtaE is required for full virulence. BtaE was immunodetected on the bacterial surface at one cell pole. Using old and new pole markers, we observed that both the BmaC and BtaE adhesins are consistently associated with the new cell pole, suggesting that, in Brucella, the new pole is functionally differentiated for adhesion. This is consistent with the inherent polarization of this bacterium, and its role in the invasion process.
Figures




Similar articles
-
The BtaF trimeric autotransporter of Brucella suis is involved in attachment to various surfaces, resistance to serum and virulence.PLoS One. 2013 Nov 13;8(11):e79770. doi: 10.1371/journal.pone.0079770. eCollection 2013. PLoS One. 2013. PMID: 24236157 Free PMC article.
-
BmaC, a novel autotransporter of Brucella suis, is involved in bacterial adhesion to host cells.Cell Microbiol. 2012 Jun;14(6):965-82. doi: 10.1111/j.1462-5822.2012.01771.x. Epub 2012 Mar 8. Cell Microbiol. 2012. PMID: 22321605
-
The BtaF Adhesin Is Necessary for Full Virulence During Respiratory Infection by Brucella suis and Is a Novel Immunogen for Nasal Vaccination Against Brucella Infection.Front Immunol. 2019 Jul 26;10:1775. doi: 10.3389/fimmu.2019.01775. eCollection 2019. Front Immunol. 2019. PMID: 31402921 Free PMC article.
-
Adhesins of Brucella: Their Roles in the Interaction with the Host.Pathogens. 2020 Nov 12;9(11):942. doi: 10.3390/pathogens9110942. Pathogens. 2020. PMID: 33198223 Free PMC article. Review.
-
The innate immune response against Brucella in humans.Vet Microbiol. 2002 Dec 20;90(1-4):383-94. doi: 10.1016/s0378-1135(02)00223-7. Vet Microbiol. 2002. PMID: 12414158 Review.
Cited by
-
Type IV secretion system of Brucella spp. and its effectors.Front Cell Infect Microbiol. 2015 Oct 13;5:72. doi: 10.3389/fcimb.2015.00072. eCollection 2015. Front Cell Infect Microbiol. 2015. PMID: 26528442 Free PMC article. Review.
-
Adhesive Functions or Pseudogenization of Type Va Autotransporters in Brucella Species.Front Cell Infect Microbiol. 2021 Apr 27;11:607610. doi: 10.3389/fcimb.2021.607610. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33987105 Free PMC article.
-
G1-arrested newborn cells are the predominant infectious form of the pathogen Brucella abortus.Nat Commun. 2014 Jul 9;5:4366. doi: 10.1038/ncomms5366. Nat Commun. 2014. PMID: 25006695 Free PMC article.
-
Non-adaptive Evolution of Trimeric Autotransporters in Brucellaceae.Front Microbiol. 2020 Nov 12;11:560667. doi: 10.3389/fmicb.2020.560667. eCollection 2020. Front Microbiol. 2020. PMID: 33281759 Free PMC article.
-
Importance of brucellosis control programs of livestock on the improvement of one health.Vet Q. 2021 Dec;41(1):137-151. doi: 10.1080/01652176.2021.1894501. Vet Q. 2021. PMID: 33618618 Free PMC article. Review.
References
-
- Thorne ET. 2001. Brucellosis, p 372–395 In Williams ES, Barker IK. (ed), Infectious diseases of wild mammals, 3rd ed Manson Publishing, London, United Kingdom
-
- Boschiroli ML, Foulongne V, O'Callaghan D. 2001. Brucellosis: a worldwide zoonosis. Curr. Opin. Microbiol. 4: 58–64 - PubMed
-
- Seleem MN, Boyle SM, Sriranganathan N. 2010. Brucellosis: a re-emerging zoonosis. Vet. Microbiol. 140: 392–398 - PubMed
-
- Celli J, Gorvel JP. 2004. Organelle robbery: Brucella interactions with the endoplasmic reticulum. Curr. Opin. Microbiol. 7: 93–97 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

