Vaccine protection against Bacillus cereus-mediated respiratory anthrax-like disease in mice
- PMID: 23319564
- PMCID: PMC3584855
- DOI: 10.1128/IAI.01346-12
Vaccine protection against Bacillus cereus-mediated respiratory anthrax-like disease in mice
Abstract
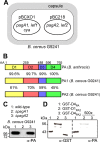
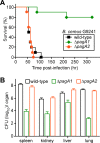
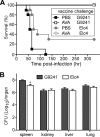
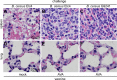
Bacillus cereus strains harboring a pXO1-like virulence plasmid cause respiratory anthrax-like disease in humans, particularly in welders. We developed mouse models for intraperitoneal as well as aerosol challenge with spores of B. cereus G9241, harboring pBCXO1 and pBC218 virulence plasmids. Compared to wild-type B. cereus G9241, spores with a deletion of the pBCXO1-carried protective antigen gene (pagA1) were severely attenuated, whereas spores with a deletion of the pBC218-carried protective antigen homologue (pagA2) were not. Anthrax vaccine adsorbed (AVA) immunization raised antibodies that bound and neutralized the pagA1-encoded protective antigen (PA1) but not the PA2 orthologue encoded by pagA2. AVA immunization protected mice against a lethal challenge with spores from B. cereus G9241 or B. cereus Elc4, a strain that had been isolated from a fatal case of anthrax-like disease. As the pathogenesis of B. cereus anthrax-like disease in mice is dependent on pagA1 and PA-neutralizing antibodies provide protection, AVA immunization may also protect humans from respiratory anthrax-like death.
Figures


Similar articles
-
Certhrax Is an Antivirulence Factor for the Anthrax-Like Organism Bacillus cereus Strain G9241.Infect Immun. 2018 May 22;86(6):e00207-18. doi: 10.1128/IAI.00207-18. Print 2018 Jun. Infect Immun. 2018. PMID: 29610258 Free PMC article.
-
Protein- and DNA-based anthrax toxin vaccines confer protection in guinea pigs against inhalational challenge with Bacillus cereus G9241.Pathog Dis. 2014 Nov;72(2):138-42. doi: 10.1111/2049-632X.12204. Epub 2014 Sep 19. Pathog Dis. 2014. PMID: 25044336
-
Two capsular polysaccharides enable Bacillus cereus G9241 to cause anthrax-like disease.Mol Microbiol. 2011 Apr;80(2):455-70. doi: 10.1111/j.1365-2958.2011.07582.x. Epub 2011 Mar 3. Mol Microbiol. 2011. PMID: 21371137 Free PMC article.
-
Molecular basis for improved anthrax vaccines.Adv Drug Deliv Rev. 2005 Jun 17;57(9):1266-92. doi: 10.1016/j.addr.2005.01.028. Epub 2005 Apr 21. Adv Drug Deliv Rev. 2005. PMID: 15935874 Review.
-
Progress and novel strategies in vaccine development and treatment of anthrax.Immunol Rev. 2011 Jan;239(1):221-36. doi: 10.1111/j.1600-065X.2010.00969.x. Immunol Rev. 2011. PMID: 21198675 Review.
Cited by
-
Capsules, toxins and AtxA as virulence factors of emerging Bacillus cereus biovar anthracis.PLoS Negl Trop Dis. 2015 Apr 1;9(4):e0003455. doi: 10.1371/journal.pntd.0003455. eCollection 2015 Apr. PLoS Negl Trop Dis. 2015. PMID: 25830379 Free PMC article.
-
Cytotoxic Potential of Bacillus cereus Strains ATCC 11778 and 14579 Against Human Lung Epithelial Cells Under Microaerobic Growth Conditions.Front Microbiol. 2016 Feb 3;7:69. doi: 10.3389/fmicb.2016.00069. eCollection 2016. Front Microbiol. 2016. PMID: 26870026 Free PMC article.
-
You Can't B. cereus - A Review of Bacillus cereus Strains That Cause Anthrax-Like Disease.Front Microbiol. 2020 Aug 19;11:1731. doi: 10.3389/fmicb.2020.01731. eCollection 2020. Front Microbiol. 2020. PMID: 32973690 Free PMC article. Review.
-
Certhrax Is an Antivirulence Factor for the Anthrax-Like Organism Bacillus cereus Strain G9241.Infect Immun. 2018 May 22;86(6):e00207-18. doi: 10.1128/IAI.00207-18. Print 2018 Jun. Infect Immun. 2018. PMID: 29610258 Free PMC article.
-
Multi-functional nano silver: A novel disruptive and theranostic agent for pathogenic organisms in real-time.Sci Rep. 2016 Sep 26;6:34058. doi: 10.1038/srep34058. Sci Rep. 2016. PMID: 27666290 Free PMC article.
References
-
- Hoffmaster AR, Ravel J, Rasko DA, Chapman GD, Chute MD, Marston CK, De BK, Sacchi CT, Fitzgerald C, Mayer LW, Maiden MC, Priest FG, Barker M, Jiang L, Cer RZ, Rilstone J, Peterson SN, Weyant RS, Galloway DR, Read TD, Popovic T, Fraser CM. 2004. Identification of anthrax toxin genes in a Bacillus cereus associated with an illness resembling inhalation anthrax. Proc. Natl. Acad. Sci. U. S. A. 101:8449–8454 - PMC - PubMed
-
- Hoffmaster AR, Hill KK, Gee JE, Marston CK, De BK, Popovic T, Sue D, Wilkins PP, Avashia SB, Drumgoole R, Helma CH, Ticknor LO, Okinaka RT, Jackson PJ. 2006. Characterization of Bacillus cereus isolates associated with fatal pneumonias: strains are closely related to Bacillus anthracis and harbor B. anthracis virulence genes. J. Clin. Microbiol. 44:3352–3360 - PMC - PubMed
-
- Wright AM, Beres SB, Consamus EN, Long SW, Flores AR, Barrios R, Richter GS, Oh SY, Garufi G, Maier H, Drews AL, Stockbauer KE, Cernoch P, Schneewind O, Olsen RJ, Musser JM. 2011. Rapidly progressive, fatal, inhalation anthraxlike infection in a human: case report, pathogen genome sequencing, pathology, and coordinated response. Arch. Pathol. Lab. Med. 135:1447–1459 - PubMed
-
- Avashia SB, Riggins WS, Lindley C, Hoffmaster A, Drumgoole R, Nekomoto T, Jackson PJ, Hill K, Williams K, Lehman L, Libal MC, Wilkins PP, Alexander J, Tvaryanas A, Betz T. 2007. Fatal pneumonia among metalworkers due to inhalation exposure to Bacillus cereus containing Bacillus anthracis toxin genes. Clin. Infect. Dis. 44:414–416 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

