Stimulation of bone formation in cortical bone of mice treated with a receptor activator of nuclear factor-κB ligand (RANKL)-binding peptide that possesses osteoclastogenesis inhibitory activity
- PMID: 23319583
- PMCID: PMC3581422
- DOI: 10.1074/jbc.M112.426080
Stimulation of bone formation in cortical bone of mice treated with a receptor activator of nuclear factor-κB ligand (RANKL)-binding peptide that possesses osteoclastogenesis inhibitory activity
Abstract
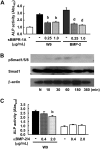
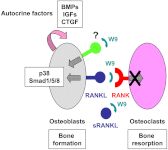
To date, parathyroid hormone is the only clinically available bone anabolic drug. The major difficulty in the development of such drugs is the lack of clarification of the mechanisms regulating osteoblast differentiation and bone formation. Here, we report a peptide (W9) known to abrogate osteoclast differentiation in vivo via blocking receptor activator of nuclear factor-κB ligand (RANKL)-RANK signaling that we surprisingly found exhibits a bone anabolic effect in vivo. Subcutaneous administration of W9 three times/day for 5 days significantly augmented bone mineral density in mouse cortical bone. Histomorphometric analysis showed a decrease in osteoclastogenesis in the distal femoral metaphysis and a significant increase in bone formation in the femoral diaphysis. Our findings suggest that W9 exerts bone anabolic activity. To clarify the mechanisms involved in this activity, we investigated the effects of W9 on osteoblast differentiation/mineralization in MC3T3-E1 (E1) cells. W9 markedly increased alkaline phosphatase (a marker enzyme of osteoblasts) activity and mineralization as shown by alizarin red staining. Gene expression of several osteogenesis-related factors was increased in W9-treated E1 cells. Addition of W9 activated p38 MAPK and Smad1/5/8 in E1 cells, and W9 showed osteogenesis stimulatory activity synergistically with BMP-2 in vitro and ectopic bone formation. Knockdown of RANKL expression in E1 cells reduced the effect of W9. Furthermore, W9 showed a weak effect on RANKL-deficient osteoblasts in alkaline phosphatase assay. Taken together, our findings suggest that this peptide may be useful for the treatment of bone diseases, and W9 achieves its bone anabolic activity through RANKL on osteoblasts accompanied by production of several autocrine factors.
Figures





Similar articles
-
Treatment of OPG-deficient mice with WP9QY, a RANKL-binding peptide, recovers alveolar bone loss by suppressing osteoclastogenesis and enhancing osteoblastogenesis.PLoS One. 2017 Sep 22;12(9):e0184904. doi: 10.1371/journal.pone.0184904. eCollection 2017. PLoS One. 2017. PMID: 28937990 Free PMC article.
-
Osteoclast regulation of osteoblasts via RANK‑RANKL reverse signal transduction in vitro.Mol Med Rep. 2017 Oct;16(4):3994-4000. doi: 10.3892/mmr.2017.7039. Epub 2017 Jul 20. Mol Med Rep. 2017. PMID: 28731168 Free PMC article.
-
Increased bone mass in mice after single injection of anti-receptor activator of nuclear factor-kappaB ligand-neutralizing antibody: evidence for bone anabolic effect of parathyroid hormone in mice with few osteoclasts.J Biol Chem. 2011 Oct 21;286(42):37023-31. doi: 10.1074/jbc.M111.246280. Epub 2011 Aug 23. J Biol Chem. 2011. PMID: 21862583 Free PMC article.
-
Osteoclast differentiation by RANKL and OPG signaling pathways.J Bone Miner Metab. 2021 Jan;39(1):19-26. doi: 10.1007/s00774-020-01162-6. Epub 2020 Oct 20. J Bone Miner Metab. 2021. PMID: 33079279 Review.
-
The Differential Effect of Metformin on Osteocytes, Osteoblasts, and Osteoclasts.Curr Osteoporos Rep. 2023 Dec;21(6):743-749. doi: 10.1007/s11914-023-00828-0. Epub 2023 Oct 5. Curr Osteoporos Rep. 2023. PMID: 37796390 Free PMC article. Review.
Cited by
-
Bone remodeling: an operational process ensuring survival and bone mechanical competence.Bone Res. 2022 Jul 18;10(1):48. doi: 10.1038/s41413-022-00219-8. Bone Res. 2022. PMID: 35851054 Free PMC article. Review.
-
RANKL/OPG; Critical role in bone physiology.Rev Endocr Metab Disord. 2015 Jun;16(2):131-9. doi: 10.1007/s11154-014-9308-6. Rev Endocr Metab Disord. 2015. PMID: 25557611 Review.
-
Peptide-Based Biomaterials for Bone and Cartilage Regeneration.Biomedicines. 2024 Jan 29;12(2):313. doi: 10.3390/biomedicines12020313. Biomedicines. 2024. PMID: 38397915 Free PMC article. Review.
-
RANKL-RANK signaling regulates osteoblast differentiation and bone formation.Bone Res. 2018 Nov 27;6:35. doi: 10.1038/s41413-018-0040-9. eCollection 2018. Bone Res. 2018. PMID: 30510840 Free PMC article. No abstract available.
-
Perforated Hydrogels Consisting of Cholesterol-Bearing Pullulan (CHP) Nanogels: A Newly Designed Scaffold for Bone Regeneration Induced by RANKL-Binding Peptides and BMP-2.Int J Mol Sci. 2022 Jul 14;23(14):7768. doi: 10.3390/ijms23147768. Int J Mol Sci. 2022. PMID: 35887115 Free PMC article.
References
-
- Yasuda H., Shima N., Nakagawa N., Yamaguchi K., Kinosaki M., Mochizuki S., Tomoyasu A., Yano K., Goto M., Murakami A., Tsuda E., Morinaga T., Higashio K., Udagawa N., Takahashi N., Suda T. (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. U.S.A. 95, 3597–3602 - PMC - PubMed
-
- Lacey D. L., Timms E., Tan H. L., Kelley M. J., Dunstan C. R., Burgess T., Elliott R., Colombero A., Elliott G., Scully S., Hsu H., Sullivan J., Hawkins N., Davy E., Capparelli C., Eli A., Qian Y. X., Kaufman S., Sarosi I., Shalhoub V., Senaldi G., Guo J., Delaney J., Boyle W. J. (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176 - PubMed
-
- Nakashima T., Hayashi M., Fukunaga T., Kurata K., Oh-Hora M., Feng J. Q., Bonewald L. F., Kodama T., Wutz A., Wagner E. F., Penninger J. M., Takayanagi H. (2011) Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 17, 1231–1234 - PubMed
-
- Nakagawa N., Kinosaki M., Yamaguchi K., Shima N., Yasuda H., Yano K., Morinaga T., Higashio K. (1998) RANK is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesis. Biochem. Biophys. Res. Commun. 253, 395–400 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

